- History & Evolution
- Biosynthesis vs. dietary uptake
- Structure and nomenclature
- Phosphatidylcholines and membrane properties
- Phosphatidylcholines and inflammation
- Phosphatidylcholines, surfactant and the lungs
- Sex differences in phosphatidylcholine levels
- Phosphatidylcholines and central nervous system infections
History and evolution
1846: discovery of lecithin (Vance 2014) | 1954: identification of pathways for biosynthesis (Zeisel 2012) | 1990s: phosphatidylcholine shown to be essential for human health
Phosphatidylcholines (PCs) are one of the most abundant glycerophospholipids found in animal and plant eukaryotic cell membranes (van der Veen et al. 2017). PCs were first identified as a component of egg yolk in 1846 by Theodore Gobley, who named them “lecithin”, after the Greek word for egg yolk (lekithos) (Vance 2014). In 1862, Adolph Strecker found that heating lecithin from bile produced a substance he called “choline”.
Lecithin was later identified as phosphatidylcholine and the two terms were often used interchangeably, though PCs are part of the broader lecithin family (Zeisel 2012).
Pathways for the biosynthesis of PCs were discovered in the 1950s. The existence of various PC molecules with fatty acyl chains of varying chain lengths and saturation statuses gave rise to a more detailed nomenclature for this family of lipids detailed below.
PCs are present in multiple tissues, including brain and nerve, and can also act as an emulsifier in the lungs. They are often referred to as membrane lipids, but animal and human studies have revealed roles for PCs in energy metabolism, lipoprotein transport and cell signaling (van der Veen et al. 2017).
Biosynthesis vs. dietary uptake
PCs are found in foods high in lecithin, such as egg yolks, soybeans, sunflower seeds, meat and fish. Dietary PC is metabolized in the small intestine by pancreatic and mucosal enzymes to generate 1-lyso-phosphatidylcholine, which then enters various pathways for lipid and glucose metabolism and fat storage (Nilsson et al. 2019).
Dietary PC is the main source of choline, an essential nutrient that supports lipid and amino acid metabolism and contributes to cell membrane structure (Tang et al. 2013). Choline also acts as precursor for the neurotransmitter acetylcholine, which supports brain and muscle function.
PCs are synthesized primarily in the endoplasmic reticulum (ER) through two main pathways: the cytidine 5-diphosphocoline (CDP-choline) or Kennedy pathway, and the phosphatidylethanolamine methyl transferase (PEMT) pathway.
In the CDP-choline pathway, cytidine triphosphate activates phosphocholine and converts it to diacylglyceride (DG) to form PC (Moessinger et al. 2014). Cytoplasmic cytidylyltransferase and cholinephosphotransferase-1 mediate the reaction (Henneberry et al. 2002). This pathway accounts for around 70% of PC synthesis in the liver (Vance 2014).
The remaining 30% of PC synthesis occurs through the PEMT pathway (Moessinger et al. 2014). Here, S-adenosyl methionine (SAM) methylates phosphatidylethanolamine (PE) to form PC. This pathway is also the main route to PC synthesis in bacteria.
Intestinal PCs, whether derived from dietary uptake, bile or de novo synthesis, are hydrolyzed by phospholipase A2 (PLA2) to lysoPCs and fatty acids, then absorbed by enterocytes (Kennelly et al. 2018). This is known as the Lands cycle. The reverse reaction is also a route to PC synthesis.
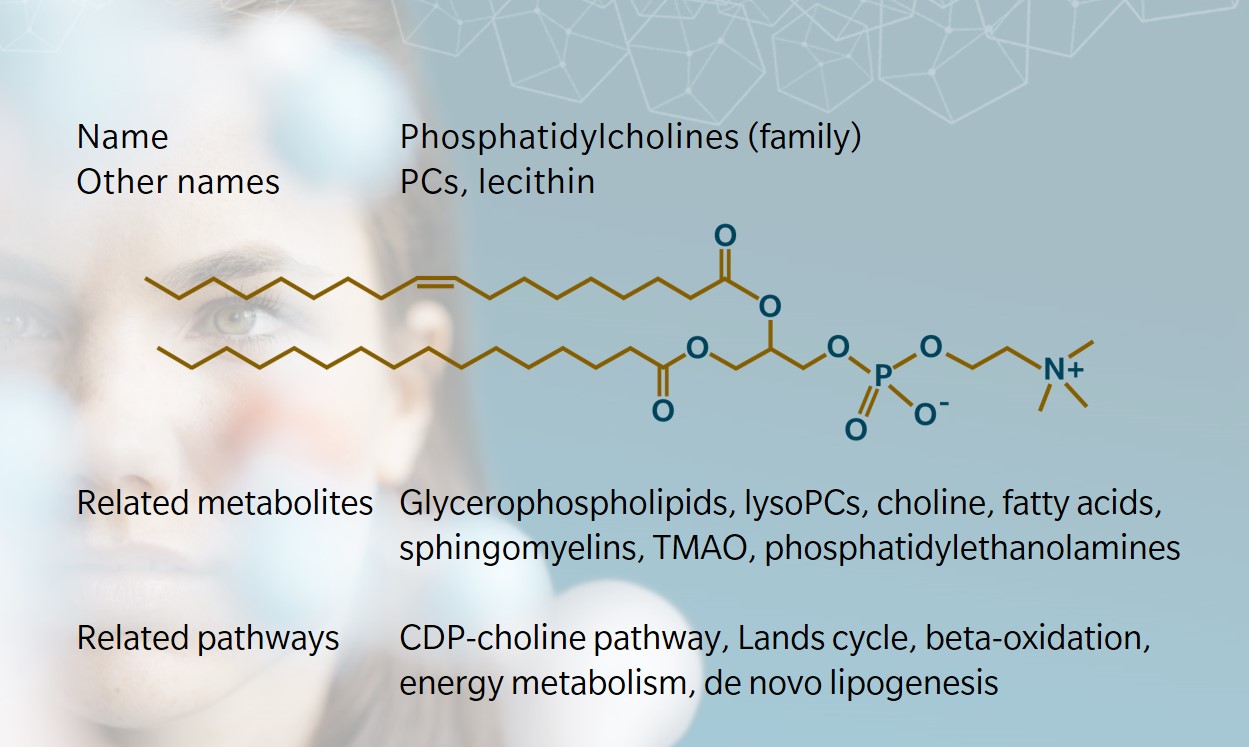
Structure and nomenclature
Phospholipids include two main categories: glycerophospholipids and sphingolipids. PCs are a member of the former, along with PEs, phosphatidylserines (PSs), phosphatidylinositols (PIs), and cardiolipins (Li et al. 2015).
PCs have a phosphocholine head group linked to two fatty acyl side chains by a glycerol backbone. They can be sub-classified into diacyls, alkylacyls or alkenylacyls, depending on the type of bond at the sn-1 position (first carbon of the glycerol backbone).
Scientific nomenclature for PCs varies. At biocrates, we use a short nomenclature where the name of the PC denotes the type of bond linking the fatty acyl groups to the glycerol backbone, and the number of carbon atoms and double bonds. For example:
- PC aa C32:1 describes a PC with two fatty acyl chains adding up to 32 carbon atoms and one double bond. The ‘aa’ indicates that both moieties at the sn-1 and sn-2 positions are fatty acyl residues bound to the glycerol backbone by ester bonds.
- PC ae C44:6 describes a PC with two fatty acyl chains adding up to 44 carbon atoms and six double bonds. The ‘ae’ denotes that one of the moieties, either in the sn-1 or sn-2 position, is a fatty alcohol residue bound by an ether bond.
- LysoPC a C18:0 describes a lysoPC with a fatty acyl chain with 18 carbon atoms and no double bonds. The ‘a’ indicates that the moiety usually at the sn-1 position is a fatty acid residue bound to the glycerol backbone by an ester bond.
Lysophosphatidylcholines are produced when one of the fatty acyl groups in PCs is removed by phospholipase.
Phosphatidylcholines and membrane properties
PCs are the most abundant phospholipid in cell membranes, accounting for 40–50% of total cellular phospholipids (van der Veen et al. 2017). Mammalian cells contain large and diverse populations of PCs, derived from the remodeling action of phospholipases and lysophospholipid acyltransferases.
PCs are commonly found in the outer layer of the cell membrane, while other glycerophospholipids (PE, PS, PI) are predominant in the inner membrane leaflet. Intracellular transport is not yet fully understood. PC-specific transfer protein and non-specific lipid transfer proteins may play a role, but animal studies suggest that neither accounts for the entirety of PC movement (Vance 2014).
PCs contribute to cell function and structure. They are central to membrane-mediated cell signaling, protein synthesis, cholesterol homeostasis and triglycerides storage and secretion (Lagace et al. 2013).
Phosphatidylcholines and inflammation
PCs are involved in the early stages of the inflammatory cascade. When PCs with a C20:4 fatty acyl group are cleaved to form lysoPCs, this releases arachidonic acid (FA (20:4)). This specific fatty acid is the precursor for a family of active lipids called eicosanoids. These include prostaglandins and leukotrienes that serve as signaling molecules during inflammation .
LysoPCs also have a role in modulating the immune response through the activation and transportation of immune cells. These functions have been associated with inflammatory diseases such as diabetes, obesity, atherosclerosis, cancer and rheumatoid arthritis (Dei Cas et al. 2020).
A high ratio of lysoPCs to PCs may indicate increased enzyme activity associated with progression of inflammatory conditions, such as osteoarthritis (Zhai et al. 2019). The amount of PC affects the size and dynamics of lipid droplets in immune cells and this variation in lipid activity can trigger stress responses. The location of PC synthesis in the ER may affect the etiology of diseases that arise from ER dysfunction (Lagace et al. 2013).
Changes in phospholipid ratios can also affect energy production and have been associated with metabolic disorders such as obesity, diabetes and atherosclerosis (van der Veen J, et al., 2017). For example, PCs are central to the established association between a PC metabolite through microbial metabolism, trimethylamine-N-oxide (TMAO), and increased risk of cardiovascular disease (Tang et al. 2013).
Phosphatidylcholines, surfactant and the lungs
PCs comprise around 80% of surfactant lipids in the lungs (Agassandian et al. 2013). The majority are in disaturated form, as dipalmitoylphosphatidylcholine (DPPC). Saturated PCs are essential components of pulmonary surfactants due to their ability to lower the surface tension on alveolar structures in the lungs, which can inhibit lung expansion and cause pulmonary edema.
This makes PCs an interesting subject for the study of respiratory disease. Surfactant proteins have been well studied as a treatment for respiratory distress syndrome in neonates (Speer et al. 2013). Efficacy of surfactant treatment in adult respiratory conditions is less well established, but studies point to a possible role in modulating the immune response in pulmonary disease (Wang et al. 2021).
Lipidomic analyses have shown links between circulating lipids, including PCs, and the severity of COVID-19 (Pimentel et al. 2021). Individuals with metabolic comorbidities have been reported to be at greater risk of more severe COVID-19. Therefore, PCs may be relevant both through their role in the immune cascade and for their surfactant properties.
Sex differences in phosphatidylcholine levels
Sex-based differences in the human blood metabolome are reasonably well established. Several metabolomic investigations have shown that women have higher levels of PCs than men (Barupal et al. 2019). A 2011 study of more than 3000 participants in the Cooperative Health Research in the Augsburg Region (KORA) cohort found sex differences for up to 78% of metabolites, including PCs (Mittelstrass et al. 2011). Concentrations of PCs were found to be significantly higher in females than in males, while the reverse was true of lysoPCs.
A 2017 study found similar results: women tended to have higher levels of PCs, while men were found to have higher concentrations of lysoPCs (Trabado et al. 2017). In the same study, older subjects were found to have higher plasma levels of PCs than younger subjects.
These findings suggest that PCs concentrations may contribute to sex differences in susceptibility for many chronic diseases.
Phosphatidylcholines and central nervous system infections
PC levels may be a useful mechanism for distinguishing bacterial and viral central nervous system (CNS) infections. A 2021 study used targeted metabolomics to develop lipid profiles for bacterial meningitis, viral meningitis or encephalitis, and noninflamed controls (Al-Mekhlafi et al. 2021). PCs were found to be significantly elevated in the cerebrospinal fluid (CSF) of patients with bacterial meningitis, compared to both viral infection and controls. Ten robust biomarkers were identified, with four of the top five PCs showing better results than standard CSF parameters.
In bacterial meningitis, changes in PCs were more strongly correlated with local CNS disease than systemic inflammation, which suggests dysfunction of the blood-CSF barrier leading to cell death.
Learn more about the roles of PCs and other phospholipids in complex chronic diseases such as cancer, Alzheimer’s disease, depression, inflammatory bowel disease, multiple sclerosis and diabetes in our whitepaper “Complex chronic diseases have a common origin”.
References
Agassandian M. et al.: Surfactant phospholipid metabolism. (2013) Biochimica et Biophysica Acta | https://doi.org//10.1016/j.bbalip.2012.09.010
Al-Mekhlafi A. et al.: Elevated Free Phosphatidylcholine Levels in Cerebrospinal Fluid Distinguish Bacterial from Viral CNS Infections.(2021) Cells | https://doi.org/10.3390/cells10051115
Barupal D. et al.: The circulating lipidome is largely defined by sex descriptors in the GOLDN, GeneBank and the ADNI studies. (2019) bioRxiv | https://doi.org/10.1101/731448
Dei Cas M. et al.: Functional Lipids in Autoimmune Inflammatory Diseases.(2020) International Journal of Molecular Sciences | https://doi.org/10.3390/ijms21093074
Henneberry A. et al.: The Major Sites of Cellular Phospholipid Synthesis and Molecular Determinants of Fatty Acid and Lipid Head Group Specificity. (2002) Molecular Biology of the Cell | https://doi.org/10.1091/mbc.01-11-0540
Kennelly J. et al.: Intestinal de novo phosphatidylcholine synthesis is required for dietary lipid absorption and metabolic homeostasis. (2018) Journal of Lipid Research | https://doi.org/10.1194/jlr.M087056
Lagace T. et al.: The role of phospholipids in the biological activity and structure of the endoplasmic reticulum. (2013) Biochimica et Biophysica Acta | https://doi.org/10.1016/j.bbamcr.2013.05.018
Li J. et al.: A review on phospholipids and their main applications in drug delivery systems. (2015) Asian Journal of Pharmaceutical Sciences | https://doi.org//10.1016/j.ajps.2014.09.004
Mittelstrass K.et al.: Discovery of Sexual Dimorphisms in Metabolic and Genetic Biomarkers. (2011) PLoS Genetics | https://doi.org/10.1371/journal.pgen.1002215
Moessinger C. et al.: Two different pathways of phosphatidylcholine synthesis, the Kennedy Pathway and the Lands Cycle, differentially regulate cellular triacylglycerol storage. (2014) BMC Cell Biology | https://doi.org/10.1186/s12860-014-0043-3
Nilsson, Å. et al.: Pancreatic and mucosal enzymes in choline phospholipid digestion. (2019) American Journal of Physiology | https://doi.org/doi.org/10.1152/ajpgi.00320.2018
Pimentel L. et al.: Cholesterol, inflammation, and phospholipids: COVID-19 share traits with cardiovascular disease. (2021) Biochimica et Biophysica Acta | https://doi.org/10.1016/j.bbalip.2020.158839
Speer C. et al.: Surfactant therapy: past, present and future. (2013) Early Human Development | https://doi.org/10.1016/S0378-3782(13)70008-2
Tang W. et al.: Intestinal Microbial Metabolism of Phosphatidylcholine and Cardiovascular Risk. (2013) The New England Journal of Medicine | https://doi.org/10.1056/NEJMoa1109400
Trabado S. et al.: The human plasma-metabolome: Reference values in 800 French healthy volunteers; impact of cholesterol, gender and age. (2017) PLoS ONE | https://doi.org/10.1371/journal.pone.0173615
van der Veen J. et al.: The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. (2017) Biochimica et Biophysica Acta | https://doi.org/10.1016/j.bbamem.2017.04.006
Vance, D.: Phospholipid methylation in mammals: from biochemistry to physiological function. (2014) Biochimica et Biophysica Acta | https://doi.org/10.1016/j.bbamem.2013.10.018
Wang S. et al.: The Role of Pulmonary Surfactants in the Treatment of Acute Respiratory Distress Syndrome in COVID-19. (2021) Frontiers in Pharmacology | https://doi.org/10.3389/fphar.2021.698905
Zeisel, S.: A brief history of choline. (2012) Annals of Nutrition and Metabolism | https://doi.org/10.1159/000343120
Zhai G. et al.: Serum lysophosphatidylcholines to phosphatidylcholines ratio is associated with symptomatic responders to symptomatic drugs in knee osteoarthritis patients. (2019) Arthritis Research & Therapy | https://doi.org/10.1186/s13075-019-2006-8