History & evolution
Biosynthesis vs. dietary uptake
Arginine and nitrogen metabolism
Arginine and neuropsychiatric disorders
Arginine and cardiometabolic diseases
Arginine and cancer
History and evolution
1886: discovered by Schulze and Steiger (Schulze et al. 1887) | 1889 : first synthesis | 1910: structure confirmed (Sørensen, 1910)
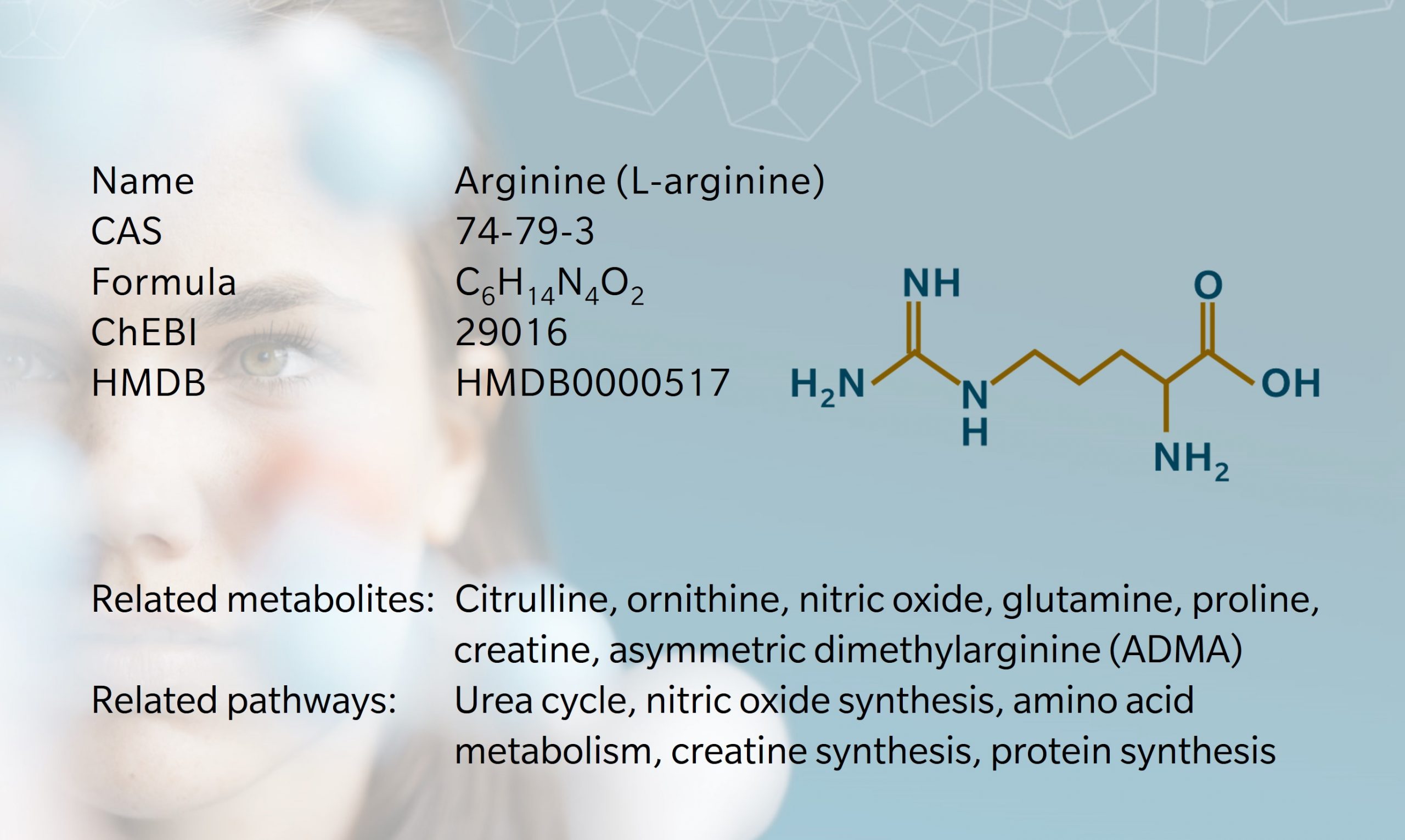
Arginine is an amino acid involved in many different metabolic processes, including protein synthesis and the synthesis of nitric oxide, urea, creatine and other metabolites (Gambardella et al, 2020). It is involved in cell division, hormone release, wound healing and T-cell function.
Arginine’s story begins in 1886, when it was first isolated from lupin seeds by Schulze and Steiger (Loscalzo, 2000). In its crystallized form, it has a silvery appearance, which inspired Schulze to name it after the Greek word for silver, árgyros (Gambardella et al, 2020). In 1895, Hedin demonstrated that arginine was a product of protein hydrolysis (Hedin, 1895). Arginine’s structure was confirmed following synthesis by Sørensen in 1910, and by the 1930s, chemists had started to understand more about its role in metabolism. When Krebs and Henseleit discovered the ornithine (urea) cycle in 1932, arginine’s role in metabolism and physiology began to be further elucidated (Wu et al., 1998).
Biosynthesis vs. dietary uptake
In humans, arginine is a semi-essential or conditionally essential amino acid (Morris, 2016). While it can be obtained from the diet, enough is produced via endogenous synthesis and protein catabolism so that in normal conditions, the body does not rely on external sources. Internally, around 80% of arginine in the body is produced through protein turnover and 15% is synthesized from citrulline in the urea cycle.
Most de novo synthesis happens via the intestinal-renal axis: citrulline is produced by epithelial cells in the small intestine, mainly from ornithine , glutamine and glutamate (Barmore et al, 2023). Citrulline is transported to the proximal tubule cells in the kidneys via blood circulation, where it is transformed into arginine by a series of enzymatic reactions involving argininosuccinate synthetase (ASS1), adenosine triphosphate (ATP) and argininosuccinate lyase. Arginine is then released back into the bloodstream. Arginine may also undergo hydrolysis by arginase, resulting in urea and ornithine. As noted, ornithine is a precursor of citrulline and may be recycled once urea is released.
Several enzymes use arginine as a substrate. Other downstream products of arginine metabolism include nitric oxide, creatine, polyamines, proline, agmatine, homoarginine, and methylated arginines (Morris, 2016). Methylated forms of arginine include asymmetric dimethyl arginine (ADMA) and its counterpart, symmetric dimethyl arginine (SDMA), which are produced during post-translational modification of proteins and released upon proteolysis.
In a Western-style diet, around 5g of arginine is consumed each day, typically from meats, dairy products, eggs, beans and nuts (Böger, 2007). Around 40% of dietary arginine is broken down in the intestine before entering the circulation (Wu et al. 1998). In healthy adults, the conversion of citrulline to arginine has been found to remain consistent regardless of arginine intake (Castillo et al, 1993).
Premature babies cannot synthesize arginine endogenously, and need to be administered arginine supplements. Adults may need supplementation or additional dietary arginine to assist in recovery from injury, burns or sepsis, or in the case of renal or intestinal dysfunction (De-Souza and Greene, 1998).
Arginine and nitrogen metabolism
Through its role in the urea cycle, arginine is a crucial player in nitrogen metabolism and the production of nitric oxide (NO) (Loscalzo, 2000). The process of metabolizing arginine into ornithine and urea helps eliminate excess nitrogen from the body. Nitric oxide synthase (NOS) released in endothelial cells metabolizes arginine to NO and citrulline (the citrulline-NO cycle). Nitric oxide is a signaling molecule with several physiological functions, including vasodilation, muscle contraction, neurotransmission and mediation of immune processes (Böger, 2007). Arginine has therefore attracted interest as a potential therapy for conditions where NO activity is compromised.
Conditions involving endothelial dysfunction, such as diabetes, hypertension and hypercholesterolemia, are often associated with a deficiency of endothelial NOS (Simon et al, 2003). Interestingly, arginine levels seem to be rate-limiting for endothelial NO synthesis, even when there is enough arginine to saturate NOS. Despite evidence that endothelial cells have multiple intracellular arginine pools, endothelial cells appear to prefer extracellular arginine. This is known as the “arginine paradox”. It could be a problem of access: one theory is that elevated ADMA concentrations inhibit NOS activity (Simon et al, 2003). Another theory is that endothelial NO production is tightly regulated by the citrulline-NO cycle (Flam et al, 2007).
Arginine and neuropsychiatric disorders
Research has shown a relationship between arginine and neuropsychiatric disorders. Patients with major depressive disorder (MDD) have been found to have elevated levels of arginine, ADMA and ornithine, as well as reduced citrulline levels and NOS activity (Loeb et al, 2020). Accumulation of ADMA in the bloodstream can be limited by the enzyme dimethylarginine dimethylaminohydrolase 1 (DDAH1), which is regulated in part by farnesoid X receptor (FXR). FXR is expressed in several tissues, including brain neurons.
In our metabolic model of MDD, we propose that in the early stages of the disease, the metabolic and microbiome environment facilitates FXR activation, DDAH1 expression and ADMA degradation, leading to elevated NO levels. In later stages of MDD, changes in bile acid levels may prompt a response to excessive NO production, which could increase ADMA levels. This would limit the amount of arginine available for NO synthesis. This model helps explain conflicting findings in depression research that show both elevated and reduced levels of NO and NOS activity in MDD (Joca et al, 2019).
Metabolomic studies have shown that selective serotonin reuptake inhibitors (SSRIs) increase arginine levels, which reduces the ADMA/arginine ratio (MahmoudianDehkordi et al, 2019). Metabolomics has also shown that ketamine activates the urea cycle, resulting in increased availability of arginine (Moaddel et al, 2018). These effects may counteract ADMA’s inhibitory effect on NOS albeit without address the high levels of ADMA. These findings suggest that ADMA plays a role in depression that has not been targeted by traditional anti-depressants and could offer a new line of treatment.
The arginine/NO pathway is also altered in Alzheimer’s disease (AD) and dementia. Targeted metabolomics has shown that levels of arginine, ADMA and citrulline are decreased in dementia, with metabolite concentrations changes associated with neurodegeneration and cognitive impairment (Fleszar et al, 2019).
Metabolomics has shown changes in arginine and polyamine metabolism in patients with mild cognitive impairment (MCI), which are associated with subsequent development of Alzheimer’s disease (AD) (Stewart et al, 2015). With blood-based metabolite profiling, MCI patients at risk of AD could be predicted up to two years earlier than conventional diagnosis. This could greatly improve AD diagnosis and enhance patient stratification in clinical trials.
Elevated arginine levels have also been identified in post-mortem studies of patients with schizophrenia (Liu et al, 2016). Arginase activity is positively associated with age of onset.
Arginine and cardiometabolic diseases
As a substrate for endothelial NO production, arginine plays a role in regulating vascular tone, making it a metabolite of interest in cardiovascular and cardiometabolic disease (Gambardella et al, 2020). A cross-sectional analysis of data from the Korean Genome and Epidemiology Study found that low arginine bioavailability and high ratios of circulating metabolites were associated with risk of metabolic syndrome (Moon et al, 2017).
Studies have shown that arginine supplementation may improve cardiovascular function and enhance insulin sensitivity, offering a potential therapy for hypertension, diabetes, obesity and metabolic syndrome (Wu and Morris, 1998). A cohort study found that high levels of dietary arginine could increase risk of type 2 diabetes, and may have a role in disease development (Mirmiran et al, 2021). Arginine supplementation has also been shown to reduce diastolic blood pressure in women with gestational hypertension (Weckman et al, 2019).
Arginine has been associated with Reynaud’s phenomenon (RP), in which patients experience a disturbed vascular response to cold temperatures (Curtiss et al, 2019). In patients with secondary RP (which is associated with autoimmune connective tissue disease), reduced endothelial NO leads to increased vasoconstriction and decreased vasorelaxation. Arginine supplementation may counteract associated elevated ADMA levels. Primary RP is not associated with elevated ADMA production, which could explain why earlier arginine trials did not show benefits in patients with primary RP.
Arginine and cancer
Arginine is well-recognized for its role in tumorigenesis, through the synthesis of NO, polyamines, nucleotides, proline and glutamate (Du and Han, 2021). Studies show a double-edged effect of arginine in cancer: it has been shown to increase tumor progression, yet also appears to be a potential candidate for cancer treatment (Du and Han, 2021).
Like leucine, arginine is an activator of the mammalian target of rapamycin (mTOR), which regulates cell proliferation and is therefore implicated in carcinogenesis. More than 70% of tumors suppress transcription of ASS1 (the enzyme responsible for arginine synthesis), rendering cancer cells dependent on exogenous arginine. Arginine deprivation is receiving growing attention as a potential therapy for several cancers (Chen et al, 2021).
As more is understood about the role of arginine and related metabolites, metabolomic profiling could be used to diagnose and screen for cancers.
Learn more about the roles of arginine in complex chronic diseases such as cancer, Alzheimer’s disease, depression, inflammatory bowel disease, multiple sclerosis and diabetes in our whitepaper “Complex chronic diseases have a common origin”.
References
Böger et al.: The Pharmacodynamics of L-Arginine. (2007) The Journal of Nutrition | https://doi.org/jn/137.6.1650S
Barmore et al.: Physiology, Urea Cycle. (2023) In StatPearls [Internet]. Treasure Island (FL): StatePearls Publishing.
Castillo et al.: Plasma arginine and citrulline kinetics in adults given adequate and arginine-free diets. (1993) Proc Natl Acad Sci USA | https://doi.org/10.1073/pnas.90.16.7749
Chen et al.: Arginine Signaling and Cancer Metabolism. (2021) Cancer (Basel) | https://doi.org/10.3390/cancers13143541
Curtiss et al.: The clinical effects of l‐arginine and asymmetric dimethylarginine: implications for treatment in secondary Raynaud’s phenomenon. (2019) J Eur Acad Dermatol Venereol | https://doi.org/10.3390/10.1111/jdv.15180
De-Souza et al.: Pharmacological Nutrition After Burn Injury. (1998) The Journal of Nutrition | https://doi.org/10.1093/jn/128.5.797
Du and Han et al.: Arginine Metabolism and Its Potential in Treatment of Colorectal Cancer. (2021) Front. Cell Dev. Biol. | https://doi.org/10.3389/fcell.2021.658861
Flam et al.: Endothelial nitric oxide production is tightly coupled to the citrulline–NO cycle. (2007) Nitric Oxide | https://doi.org/10.1016/j.niox.2007.07.001
Fleszar et al.: Targeted metabolomic analysis of nitric oxide/L-arginine pathway metabolites in dementia: association with pathology, severity, and structural brain changes. (2019) Scientific Reports | https://doi.org/10.1038/s41598-019-50205-0
Gambardella et al.: Arginine and Endothelial Function. (2020) Biomedicines | https://doi.org/10.3390/biomedicines8080277
Hedin et al.: Über die Bildung von Arginin aus Proteinkörper. (1895) Z Physiol Chem | https://doi.org/10.1515/bchm2.1896.21.2-3.155
Joca et al.: Nitric oxide signaling and antidepressant action revisited. (2019) Cell Tissue Res | https://doi.org/10.1007/s00441-018-02987-4
Liu et al.: Altered brain arginine metabolism in schizophrenia. (2016) Transl Psychiatry | https://doi.org/10.1038/tp.2016.144
Loeb et al.: Nitric Oxide Synthase activity in major depressive episodes before and after antidepressant treatment: Results of a large case-control treatment study. (2020) Psychol Med | https://doi.org/10.1017/S0033291720001749
Loscalzo et al.: What We Know and Don’t Know About l-Arginine and NO. (2000) Circulation | https://doi.org/10.1161/01.CIR.101.18.2126
MahmoudianDehkordi et al.: Altered bile acid profile associates with cognitive impairment in Alzheimer’s disease – An emerging role for gut microbiome. (2019) Alzheimers Dement | https://doi.org/10.1016/j.jalz.2018.07.217
Mirmiran et al.: Habitual intake of dietary L-arginine in relation to risk of type 2 diabetes: a prospective study. (2021) BMC Endocr Disord | https://doi.org/10.1186/s12902-021-00774-x
Moaddel et al.: Plasma metabolomic profiling of a ketamine and placebo crossover trial of major depressive disorder and healthy control subjects. (2018) Psychopharmacology (Berl) | https://doi.org/10.1007/s00213-018-4992-7
Moon et al.: Alterations in Circulating Amino Acid Metabolite Ratio Associated with Arginase Activity Are Potential Indicators of Metabolic Syndrome: The Korean Genome and Epidemiology Study. (2017) Nutrients | https://doi.org/10.3390/nu9070740
Morris et al.: Arginine Metabolism Revisited. (2016) The Journal of Nutrition | https://doi.org/10.3945/jn.115.226621
Sørensen et al.: Über die Synthese des dl-Arginins (α-amino-δ-guanidino-n-Valeriansaure) und der Isomeren δ-guanidino-α-amino-n-Valeriansaure). (1910) Chem Ber | https://doi.org/10.1002/cber.191004301109
Schulze and Steiger et al.: Ueber das Arginin [On arginine]. (1887) Zeitschrift für Physiologische Chemie | https://babel.hathitrust.org/cgi/pt?id=coo.31924078260597&view=1up&seq=55
Scull and Rose et al.: Arginine metabolism: i. The relation of the arginine content of the diet to the increments in tissue arginine during growth. (1930) Journal of Biological Chemistry | https://doi.org/10.1016/S0021-9258(18)76725-2
Simon et al.: Role of neutral amino acid transport and protein breakdown for substrate supply of nitric oxide synthase in human endothelial cells. (2003) Circ Res | https://doi.org/10.1161/01.RES.0000097761.19223.0D
Stewart et al.: Untargeted Metabolomic Analysis of Human Plasma Indicates Differentially Affected Polyamine and L-Arginine Metabolism in Mild Cognitive Impairment Subjects Converting to Alzheimer’s Disease. (2015) PLoS ONE | https://doi.org/10.1371/journal.pone.0119452
Weckman et al.: Perspective: L-arginine and L-citrulline Supplementation in Pregnancy: A Potential Strategy to Improve Birth Outcomes in Low-Resource Settings. (2019) Adv Nutr | https://doi.org/10.1093/advances/nmz015
Wu and Morris et al.: Arginine metabolism: nitric oxide and beyond. (1998) Biochem J. | https://doi.org/10.1042/bj3360001