- History & Evolution
- Biosynthesis vs. dietary uptake
- ADMA and NOS inhibition
- ADMA, the endothelium and cardiometabolic diseases
- ADMA, neurology, and depression
History and evolution
1970: first identified in human urine (Kakimoto & Akazawa 1970) | 1992: discovery of ADMA’s effect on NOS (Leone et al. 1992)
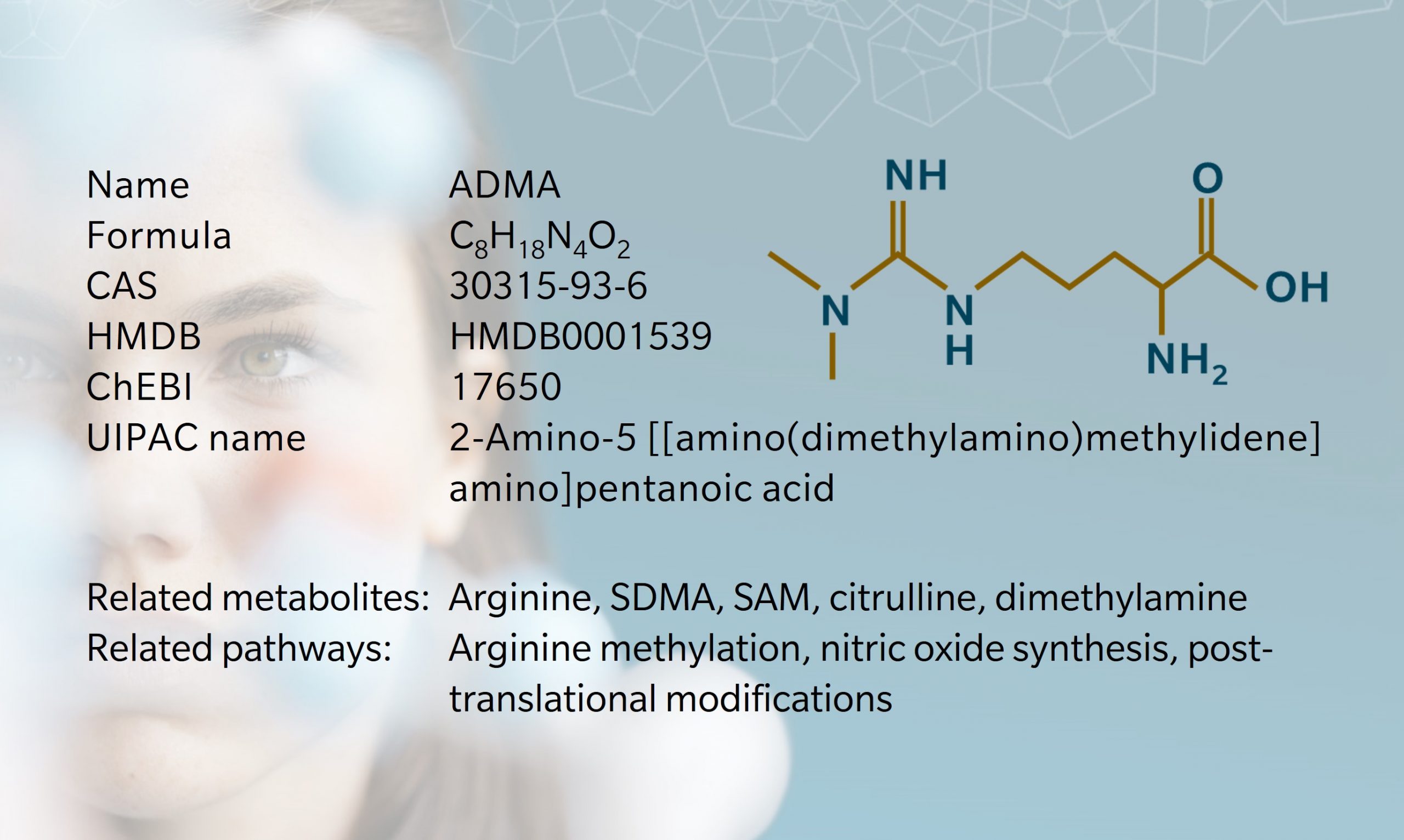
Asymmetric dimethyl arginine (known as ADMA) is one of several methylated forms of the amino acid arginineproduced during post-translational modification of proteins and released upon proteolysis. These methylated arginines are non-proteinogenic, which means that they cannot be used to build new proteins; they constitute a net “loss” of arginine for this purpose. However, this doesn’t mean, that they are inactive. As we will see, ADMA is a particularly potent inhibitor of neurological and cardiovascular processes.
ADMA, and its counterpart symmetrical dimethyl arginine (SDMA) were first identified in an analysis of human urine by Kakimoto and Akazawa (1970). At the time, the closest relative to these molecules was asterubin, a compound found in starfish. The authors went on to detect ADMA and SDMA in the urine and in several organs of rats.
Biosynthesis vs. dietary uptake
About 300 mg of free ADMA are released daily in our blood stream through cellular metabolism (Achan et al. 2003). Throughout the life cycle of cells, proteins are formed and degraded. Arginine methylation is a form of post-translational modification that serves functions including interaction of the protein with nucleic acids (either DNA or RNA), for instance in histones (Blanc and Richard 2017).
ADMA is characterized by two methylations found on a single nitrogen atom and introduced by enzymes of the protein arginine N-methyltransferase (PRMT) family (Morales et al. 2016). The methyl donor for the reaction is S-adenosylmethionine (SAM), also known as a precursor of spermidine and other polyamines.
Owing to its close structural proximity to arginine, ADMA competes with arginine, both for transporters (such as cationic amino acid transporters (CAT)) and for enzyme catalytic centers (by allosteric competition) (Bode-Böger et al. 2007, Teerlink et al. 2009). Thus, ADMA is far from being an idle waste product of proteolysis and requires adequate removal to avoid detrimental effects.
In humans, about 20% of the free ADMA is excreted in urine (Achan et al. 2003). Under physiological conditions, the remaining 80% is degraded by dimethylarginine dimethylaminohydrolase (DDAH) enzymes that generate citrulline and dimethylamine or by alanine-glyoxylate aminotransferase 2 (AGTX2).
This process can take place in several organs, including the kidneys, brain and liver (Tain et al. 2017). In contrast, SDMA is almost exclusively cleared by excretion in the urine. Both ADMA and SDMA are considered uremic toxins, as they can become harmful when proper renal clearance is impaired (Vanholder et al. 2003).
ADMA and nitric oxide synthase (NOS) inhibition
Two decades after discovering ADMA, scientists began to understand its role in inhibiting the enzyme NOS (Leone et al. 1992). This opened the door for ADMA investigations in specialties where NOS plays an important role, such as cardiovascular and neurological research.
NOS converts arginine to citrulline, releasing the signaling molecule nitric oxide (NO). Three isoforms of the enzyme exist in humans. Neuronal NOS (nNOS) is found primarily in nervous tissue. Endothelial NOS (eNOS) is found in endothelial cells. Inducible NOS (iNOS) is found in liver and muscle tissue, and in immune cells. nNOS and eNOS are constitutively expressed, while iNOS expression requires signaling, for example by cytokines.
In the liver, iNOS catalyzes the opposite reaction (arginine to citrulline) of urea cycle enzymes (citrulline to arginine). Thus, a disruption of iNOS by locally elevated ADMA levels can potentially affect the urea cycle, with consequences on polyamine metabolism and nitrogen excretion.
While nNOS and eNOS are constitutively expressed, iNOS expression requires a stimulus such as cytokine signaling. ADMA is a potent inhibitor of nNOS and eNOS, and has a milder effect on iNOS (reviewed by Palm et al. 2007). SDMA is also an inhibitor of NOS, although less studied than ADMA (Bode-Böger et al. 2006).
ADMA, the endothelium and cardiometabolic diseases
Owing to its expresseion in endothelial cells (the cells that form blood vessels), eNOS is found where blood supply takes place in and around many organs. In plasma membranes, eNOS is particularly prevalent in cholesterol- and sphingolipid-rich areas. It binds calmodulin in a calcium-dependent manner upon activation by several factors such as vascular endothelial growth factor (VEGF) (Tran et al. 2016). The NO generated by eNOS is essential to vascular relaxation and platelet aggregation, and has been linked to cardiovascular protection.
Elevated ADMA levels are associated with several cardiometabolic diseases including hypertension, peripheral arterial occlusive disease, congestive heart failure, congenital heart disease, coronary artery disease, deep vein thrombosis and diabetes mellitus (as reviewed by Tain & Hsu 2017).
The administration of ADMA in the artery of healthy volunteers allowed to reproduce several symptoms of patients with CVD associated with high ADMA levels: high blood pressure, endothelial dysfunction, decreased cardiac output (Achan et al. 2003).
ADMA, neurology, and depression
nNOS has several functions in the brain via NO signaling. These include synaptic plasticity, long-term potentiation, and vascular tone regulation. In neurons, nNOS is coupled to the N-methyl-D-aspartate (NMDA)-receptor via calcium signaling. As a result, nNOS activation is also modulated by NMDA ligands that include the kynurenine metabolites quinolinic acid (activating but also neurotoxic) and kynurenic acid (inhibiting and considered neuroprotective) (reviewed by Reus et al. 2015).
Several neurological and psychological disorders have been associated with elevated ADMA levels in case/control studies. These include Parkinson’s disease, stroke, and schizophrenia (as reviewed by Tain & Hsu 2017).
In major depressive disorder (MDD), NOS activity is often altered, although the direction of change varies between studies (reviewed by Joca et al. 2019)). Several case/control studies of MDD demonstrated an increase in circulating ADMA levels (Baranyi et al. 2015, Loeb et al. 2021), with the notable exception of a study by Ozden et al. 2020).
Interestingly, several treatments of depression that improved symptoms, did not affect ADMA levels but increased the levels of circulating arginine, thus raising the arginine/ADMA ratio (MahmoudianDehkordi et al. 2021; Moaddel et al. 2018). These findings suggest that several lines of depression treatment could be restoring NOS activity. This seems to happen not through fixing the problem of elevated levels of NOS inhibitor, but by cincumventing it and winning the allosteric competition against ADMA.
I discuss this topic in more detail, along with external determinants of depression, in our webinar on deciphering the etiology of depression using metabolomics.
Learn more about the roles of ADMA and other arginine metabolites in complex chronic diseases such as cancer, Alzheimer’s disease, depression, inflammatory bowel disease, multiple sclerosis and diabetes in our whitepaper “Complex chronic diseases have a common origin”.
References
Achan V. et al.: Asymmetric dimethylarginine causes hypertension and cardiac dysfunction in humans and is actively metabolized by dimethylarginine dimethylaminohydrolase. (2003) Arteriosclerosis, thrombosis, and vascular biology | https://doi.org/10.1161/01.ATV.0000081742.92006.59
Baranyi, A. et al.: Nitric Oxide-Related Biological Pathways in Patients with Major Depression. (2015) PLOS ONE | https://doi.org/10.1371/journal.pone.0143397
Blanc R. et al.: Arginine Methylation: The Coming of Age. (2017) Molecular cell | https://doi.org/10.1016/j.molcel.2016.11.003
Bode-Böger S. et al.: The L-arginine paradox: Importance of the L-arginine/asymmetrical dimethylarginine ratio. (2007) Pharmacology & Therapeutics | https://doi.org/10.1016/j.pharmthera.2007.03.002
Bode-Böger, S. et al.: Symmetrical dimethylarginine: a new combined parameter for renal function and extent of coronary artery disease. (2006) Journal of the American Society of Nephrology | https://doi.org/10.1681/ASN.2005101119
Joca S. et al.: Nitric oxide signalling and antidepressant action revisited. (2019) Cell Tissue Res | https://doi.org/10.1007/s00441-018-02987-4
Kakimoto Y. et al.: Isolation and identification of N-G,N-G- and N-G,N’-G-dimethyl-arginine, N-epsilon-mono-, di-, and trimethyllysine, and glucosylgalactosyl- and galactosyl-delta-hydroxylysine from human urine. (1970) Journal of Biological Chemistry | https://pubmed.ncbi.nlm.nih.gov/5472370
Leone, A. et al.: Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. (1992) Lancet | https://doi.org/10.1016/0140-6736(92)90865-z
Loeb E. et al.: Is the decrease in NOx due to a lack of substrate or a NOS inhibition in patients with major depression? : Commentary on Hess et al. 2017. (2021) Psychopharmacology | https://doi.org/10.1007/s00213-020-05747-x
MahmoudianDehkordi S. et al.: Alterations in acylcarnitines, amines, and lipids inform about the mechanism of action of citalopram/escitalopram in major depression. (2021) Transl Psychiatry | https://doi.org/10.1038/s41398-020-01097-6
Moaddel R. et al.: Plasma metabolomic profiling of a ketamine and placebo crossover trial of major depressive disorder and healthy control subjects. (2018) Psychopharmacology | https://doi.org/10.1007/s00213-018-4992-7
Morales Y. et al.: (2016): Biochemistry and regulation of the protein arginine methyltransferases (PRMTs). (2016) Archives of Biochemistry and Biophysics | https://doi.org/10.1016/j.abb.2015.11.030
Ozden A. et al.: Altered plasma levels of arginine metabolites in depression. (2020) Journal of psychiatric research | https://doi.org/10.1016/j.jpsychires.2019.10.004
Palm F.: Dimethylarginine dimethylaminohydrolase (DDAH): expression, regulation, and function in the cardiovascular and renal systems. (2007) American journal of physiology. Heart and circulatory physiology | https://doi.org/10.1152/ajpheart.00998.2007
Réus G. et al.: Kynurenine pathway dysfunction in the pathophysiology and treatment of depression: Evidences from animal and human studies. (2015) Journal of psychiatric research | https://doi.org/10.1016/j.jpsychires.2015.05.007
Tain Y. et al.: Toxic Dimethylarginines: Asymmetric Dimethylarginine (ADMA) and Symmetric Dimethylarginine (SDMA). (2017) Toxins | https://doi.org/10.3390/toxins9030092
Teerlink T. et al.: Cellular ADMA: regulation and action. (2009) In Pharmacological Research | https://doi.org/10.1016/j.phrs.2009.08.002
Tran J. et al.: Activation of Endothelial Nitric Oxide (eNOS) Occurs through Different Membrane Domains in Endothelial Cells. (2016) In PLOS ONE | https://doi.org/10.1371/journal.pone.0151556
Vanholder R. et al.: Review on uremic toxins: classification, concentration, and interindividual variability. (2003) Kidney international | https://doi.org/10.1046/j.1523-1755.2003.00924.x