- History & Evolution
- Biosynthesis
- Spermidine, autophagy and aging
- Spermidine and cancer
- Spermidine and neurology
- Spermidine and hair
- References
History & Evolution
1924: first synthesis by Otto Rosenheim (Bachrach 2010)
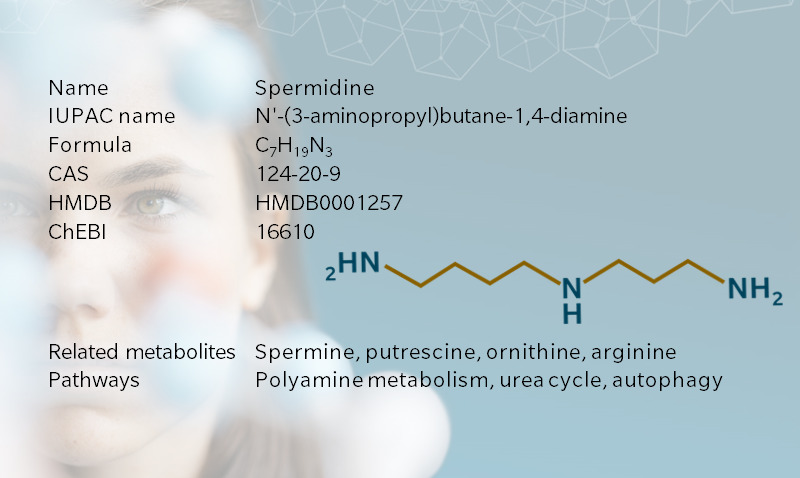
Once considered a uremic toxin, spermidine has been put in a more positive light by recent research pointing to its role in cell homeostasis, cancer treatment, longevity, and hair growth. Spermidine is a biogenic amine of the polyamine family, which contribute to cell division and growth. It’s also the downstream metabolite of putrescine, which is associated with bodily odors. Spermidine and its precursor, spermine, get their name from semen, after crystals of spermine were first identified in human semen in the seventeenth century (Bachrach 2010).
Biosynthesis and dietary uptake
Spermidine is present in large amounts in mammals. It can also be readily absorbed in the small intestine from foods such as wheat germ, soy bean, aged cheese and mushrooms (Bardócz et al. 1995). In humans, it can be synthesized by commensal bacteria in the large intestine from putrescine or arginine (Matsumoto et al. 2012; Hanfrey et al. 2011; Tofalo et al. 2019). Mammalian cells are also capable of synthesizing spermidine from putrescine, itself a product of the metabolism of arginine, linking polyamines to the urea cycle (Madeo et al. 2018). In humans, circulating levels of spermidine are often in the low micromolar range, although they show a strong inter-individual variability. This is most likely due to the effect of diet on overall spermidine concentration (Soda et al. 2009).
Spermidine, autophagy and aging
In the last decade, spermidine has attracted interest as a promoter of longevity. In 2009, Eisenberg et al. linked spermidine to increased lifespan, reduced oxidative stress, and reduced necrosis across species. The effect of spermidine on acetylation of proteins and chromatin was identified as a key mechanism for the modulation of autophagy, increasing the lifespan of yeast, flies, worms, and human cells (Eisenberg et al. 2009). Autophagy, the mechanism by which cells remove malfunctioning proteins and organelles, is an essential player in cellular homeostasis. This makes it a major target of drug development for conditions as varied as cancer, Alzheimer’s disease, and cardiovascular diseases (Onorati et al. 2018; Ren et al. 2018; Uddin et al. 2018). As an endogenous activator of autophagy, spermidine has attracted a lot of attention for dietary intervention and drug development.
Spermidine and cancer
Polyamines were originally relevant to cancer research because of their role in cell proliferation and growth. Disturbance of polyamine metabolism was observed in several types of cancer, including skin, breast, lung, prostate, and colon (Madeo et al. 2018; Nowotarski et al. 2013). However, more recent cancer research studies have focused on spermidine as a caloric restriction mimetic (CRM) with a positive impact. CRMs are molecules that limit cancer cells’ access to nutrients, thus making them more vulnerable to anti-cancer treatment. As an autophagy activator, spermidine has potential for both cancer prevention and treatment, especially to limit tumor growth (Fan et al. 2020; Kocaturk et al. 2019). In 2018, a prospective population-based study linked high levels of spermidine intake to lower mortality, including mortality from cancer (Kiechl et al. 2018). Researchers are therefore exploring both spermidine supplementation and more elaborate treatments such as the use of spermidine analogues targeting the DNA of tumor cells (Wang et al. 2018).
Spermidine and neurology
Circulating spermidine levels decrease with age (Pekar et al. 2020). This, and the fact that autophagy is of interest in the treatment of neurodegenerative diseases (Maria De Risi et al. 2020), makes spermidine an interesting candidate for further investigation. In patients with Parkinson’s disease (PD), the spermine to spermidine ratio in plasma was strongly decreased, suggesting an inhibition of the enzyme spermine synthase (Saiki et al. 2019). Interestingly, PD patients were found to have elevated levels of acetylated forms of spermidine and putrescine, and a general hyperacetylation consistent with autophagy activation. In a randomized controlled Phase IIa trial, supplementation with spermidine showed a positive impact on memory performance after just three months of administration in older adults at risk of dementia (Wirth et al. 2018). In the senescence accelerated mouse model SAMP8, spermidine was shown to slow neurodegeneration, restoring impaired mitochondrial function and reducing inflammation (Xu et al. 2020). Spermine and rapamycin (a classical activator of autophagy via the mTOR pathway), induced similar effects, although spermine tended to be less effective.
Spermidine and hair
A more exotic field for the application of spermidine research is hair growth and hair loss. In vitro studies on human scalp and hair follicle epithelial stem cells revealed the effect of spermidine on hair growth, but also on the regulation of epithelial stem cells (Ramot et al. 2011). A 90-day study showed that spermidine supplementation promoted hair growth and resistance in human subjects (Rinaldi et al. 2017). Immunohistochemical staining in rat hair follicles revealed the distribution patterns of putrescine, spermidine and spermine in the regions most linked to hair growth (Yamamoto et al. 2018). However, only the polyamines were present in high amounts in the epidermis and fibroblasts.
References
Bachrach, U: The early history of polyamine research (2010) Plant Physiology and Biochemistry | doi: 10.1016/j.plaphy.2010.02.003
Bardócz; Tracey J. Duguid; David S. Brown; George Grant; Arpad Pusztai; Ann White; Ann Ralph: The importance of dietary polyamines in cell regeneration and growth (1995) British Journal of Nutrition | doi: 10.1079/BJN19950087
Eisenberg, T; Knauer, H; Schauer, A; Büttner, S; Ruckenstuhl, C; Carmona-Gutierrez, D; Ring, J; Schroeder, S; Magnes, C; Antonacci, L; Fussi, H; Deszcz, L; Hartl, R; Schraml, E; Criollo, A; Megalou, E; Weiskopf, D; Laun, P; Heeren, G; Breitenbach, M; Grubeck-Loebenstein, B; Herker, E; Fahrenkrog, B; Fröhlich, K-U; Sinner, F; Tavernarakis, N; Minois, N; Kroemer, G; Madeo, F: Induction of autophagy by spermidine promotes longevity (2009) Nature cell biology | doi: 10.1038/ncb1975
Fan, J; Feng, Z; Chen, N: Spermidine as a target for cancer therapy (2020) Pharmacological Research | doi: 10.1016/j.phrs.2020.104943
Hanfrey C; Bruce M. Pearson; Stuart Hazeldine; Jeongmi Lee; Duncan J. Gaskin; Patrick M. Woster; Margaret A. Phillips; Anthony J. Michael: Alternative Spermidine Biosynthetic Route Is Critical for Growth of Campylobacter jejuni and Is the Dominant Polyamine Pathway in Human Gut Microbiota * (2011) Journal of Biological Chemistry | doi: 10.1074/jbc.M111.307835
Kiechl, S; Pechlaner, R; Willeit, P; Notdurfter, M; Paulweber, B; Willeit, K; Werner, P; Ruckenstuhl, C; Iglseder, B; Weger, S; Mairhofer, B; Gartner, M; Kedenko, L; Chmelikova, M; Stekovic, S; Stuppner, H; Oberhollenzer, F; Kroemer, G; Mayr, M; Eisenberg, T; Tilg, H; Madeo, F; Willeit, J: Higher spermidine intake is linked to lower mortality: a prospective population-based study (2018) The American journal of clinical nutrition | doi: 10.1093/ajcn/nqy102
Kocaturk, NM; Akkoc, Y; Kig, C; Bayraktar, O; Gozuacik, D; Kutlu, O: Autophagy as a molecular target for cancer treatment (2019) European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences | doi: 10.1016/j.ejps.2019.04.011
Madeo F; Tobias Eisenberg; Federico Pietrocola; Guido Kroemer: Spermidine in health and disease (2018) Science | doi: 10.1126/science.aan2788
De Risi M; Giulia Torromino; Michele Tufano; Stéphanie Moriceau; Annabella Pignataro; Manon Rivagorda; Nicolò Carrano; Silvia Middei; Carmine Settembre; Martine Ammassari‐Teule; Fabrizio Gardoni; Andrea Mele; Franck Oury; Elvira De Leonibus: Mechanisms by which autophagy regulates memory capacity in ageing (2020) Aging Cell | doi: 10.1111/acel.13189
Matsumoto M; Ryoko Kibe; Takushi Ooga; Yuji Aiba; Shin Kurihara; Emiko Sawaki; Yasuhiro Koga; Yoshimi Benno: Impact of Intestinal Microbiota on Intestinal Luminal Metabolome (2012) Scientific Reports | doi: 10.1038/srep00233
Nowotarski SL; Patrick M. Woster; Robert A. Casero: Polyamines and cancer: implications for chemotherapy and chemoprevention (2013) Expert Reviews in Molecular Medicine | doi: 10.1017/erm.2013.3
Onorati, AV; Dyczynski, M; Ojha, R; Amaravadi, RK: Targeting autophagy in cancer (2018) Cancer | doi: 10.1002/cncr.31335
Pekar T; Aribert Wendzel; Walter Flak; Alexandra Kremer; Susanne Pauschenwein-Frantsich; Anna Gschaider; Felix Wantke; Reinhart Jarisch: Spermidine in dementia (2020) Wiener klinische Wochenschrift | doi: 10.1007/s00508-019-01588-7
Ramot, Y; Tiede, S; Bíró, T; Abu, BMH; Sugawara, K; Philpott, MP; Harrison, W; Pietilä, M; Paus, R: Spermidine promotes human hair growth and is a novel modulator of human epithelial stem cell functions (2011) PloS one | doi: 10.1371/journal.pone.0022564
Ren, J; Sowers, JR; Zhang, Y: Metabolic Stress, Autophagy, and Cardiovascular Aging: from Pathophysiology to Therapeutics (2018) Trends in endocrinology and metabolism: TEM | doi: 10.1016/j.tem.2018.08.001
Rinaldi, F; Marzani, B; Pinto, D; Ramot, Y: A spermidine-based nutritional supplement prolongs the anagen phase of hair follicles in humans: a randomized, placebo-controlled, double-blind study (2017) Dermatology practical & conceptual | doi: 10.5826/dpc.0704a05
Saiki, S; Sasazawa, Y; Fujimaki, M; Kamagata, K; Kaga, N; Taka, H; Li, Y; Souma, S; Hatano, T; Imamichi, Y; Furuya, N; Mori, A; Oji, Y; Ueno, SI; Nojiri, S; Miura, Y; Ueno, T; Funayama, M; Aoki, S; Hattori, N: A metabolic profile of polyamines in parkinson disease: A promising biomarker (2019) Annals of neurology | doi: 10.1002/ana.25516 .
Soda; Yoshihiko KANO; Masako SAKURAGI; Koichi TAKAO; Alan LEFOR; Fumio KONISHI: Long-Term Oral Polyamine Intake Increases Blood Polyamine Concentrations (2009) Journal of Nutritional Science and Vitaminology | doi: 10.3177/jnsv.55.361
Tofalo, R; Cocchi, S; Suzzi, G: Polyamines and Gut Microbiota (2019) Frontiers in Nutrition | doi: 10.3389/fnut.2019.00016
Uddin, MS; Stachowiak, A; Mamun, AA; Tzvetkov, NT; Takeda, S; Atanasov, AG; Bergantin, LB; Abdel-Daim, MM; Stankiewicz, AM: Autophagy and Alzheimer’s Disease: From Molecular Mechanisms to Therapeutic Implications (2018) Frontiers in aging neuroscience | doi: 10.3389/fnagi.2018.00004
Wang, D; Liu, Q; Di Wu; He, B; Li, J; Mao, C; Wang, G; Qian, H: Isothermal Self-Assembly of Spermidine-DNA Nanostructure Complex as a Functional Platform for Cancer Therapy (2018) ACS applied materials & interfaces | doi: 10.1021/acsami.8b03464
Wirth, M; Benson, G; Schwarz, C; Köbe, T; Grittner, U; Schmitz, D; Sigrist, SJ; Bohlken, J; Stekovic, S; Madeo, F; Flöel, A: The effect of spermidine on memory performance in older adults at risk for dementia: A randomized controlled trial (2018) Cortex; a journal devoted to the study of the nervous system and behavior | doi: 10.1016/j.cortex.2018.09.014
Xu, T-T; Li, H; Dai, Z; Lau, GK; Li, B-Y; Zhu, W-L; Liu, X-Q; Liu, H-F; Cai, W-W; Huang, S-Q; Wang, Q; Zhang, S-J: Spermidine and spermine delay brain aging by inducing autophagy in SAMP8 mice (2020) Aging | doi: 10.18632/aging.103035
Yamamoto; Takamitsu Makino; Hideo Kudo; Hironobu Ihn; Yasuko Murakami; Senya Matsufuji; Kunio Fujiwara; Masashi Shin: Expression and distribution patterns of spermine, spermidine, and putrescine in rat hair follicle (2018) Histochemistry and Cell Biology | doi: 10.1007/s00418-017-1621-1