- History & evolution
- Biosynthesis vs. dietary uptake
- Phosphatidylethanolamines and membranes
- Phosphatidylethanolamines, lipoproteins and liver disease
- Phosphatidylethanolamines, neurology and aging
History and evolution
1884: isolation from brain by Thudichum | | 1952: PE structure by Baer et al. (Vance et al. 2013)
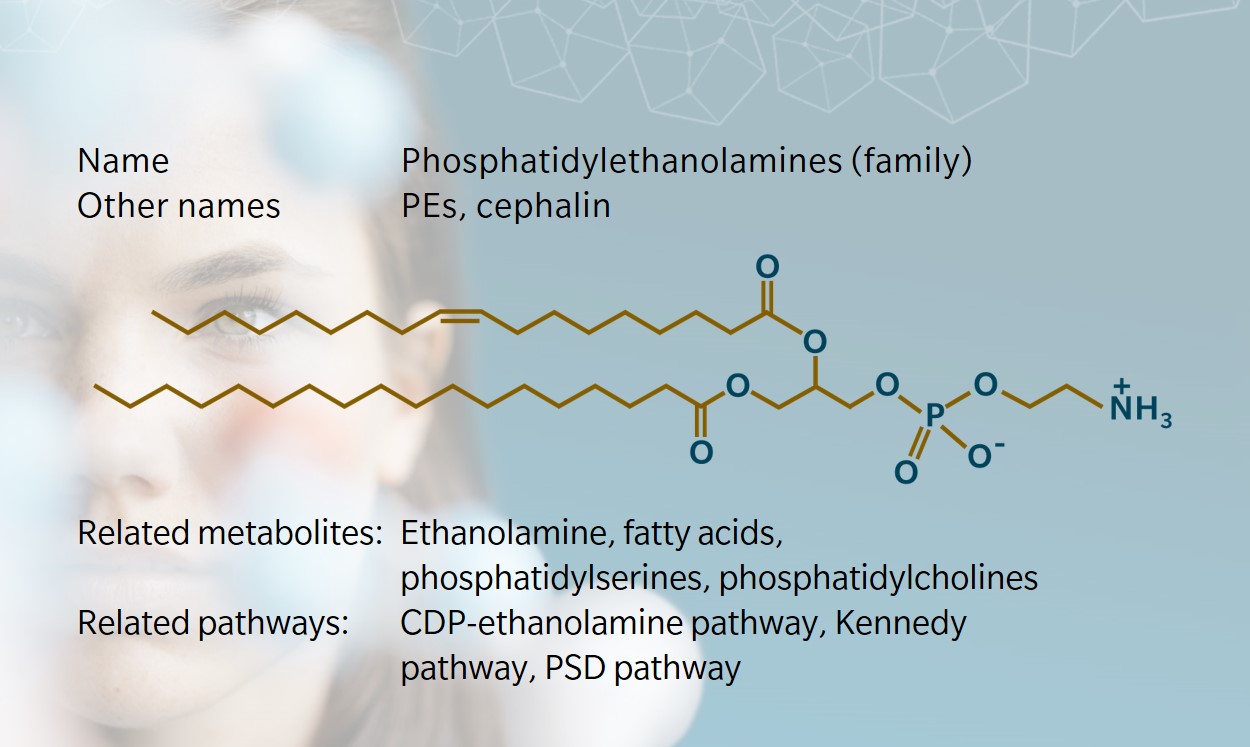
Phosphatidylethanolamine (PE) is a sub-class of phospholipids with a variety of functions in animals, plants and microorganisms. Like other phospholipids, PEs are more than simply the building blocks of membranes: their functions range from regulation of membrane fluidity to modulation of intracellular signaling, protein folding and post-translational modification. Together with phosphatidylserines (PSs), PEs were first isolated from brain tissue in 1884.
They were originally referred to as “cephalin”, deriving their name from the Greek word kephal, meaning head. In the brain, PEs constitute about 45% of all phospholipids. Today, we know that PEs come in many sizes (chain lengths of their fatty acyl groups) and shapes (number of unsaturations) and serve a variety of functions.
Biosynthesis vs. uptake
PEs consist of a glycerol backbone esterified in three positions by two fatty acids and a phosphoric acid. In the head group of this phospholipid, the phosphate group is combined to the small molecule ethanolamine. The latter is sourced from diet and found free in micromolar concentrations in biofluids in mammals (Patel et al. 2017; Vance et al. 2013). PEs are the main phospholipid in egg yolk.
In humans, PEs can be absorbed through the intestine (Cohn et al. 2010; Ikuo et al. 1987). Two main pathways lead to de novo PE synthesis in two sub-cellular compartments (reviewed by Vance et al.2013):
- the Kennedy pathway, with one branch yielding phosphatidylcholines (PCs) and one yielding PEs, which takes place in the endoplasmic reticulum (ER)
- the phosphatidylserine decarboxylation (PSD) pathway, which uses PSs as precursor for PE synthesis and takes place in the mitochondria.
In the ER, PEs are formed in the cytidine diphosphate (CDP)-ethanolamine branch of the Kennedy pathway. A diglyceride (glycerol already bound to two fatty acyl groups) is combined to the ethanolamine group provided by CDP-ethanolamine, producing PE and CDP. The most commonly described rate-limiting step for this pathway takes place in the cytosol; i.e. the conversion of ethanolamine phosphate to CDP-ethanolamine by the enzyme cytidine triphosphate (CTP):phosphoethanolamine cytidylyltransferase.
In the outer aspect of the inner mitochondrial membrane, the PSD pathway converts PSs to PEs in a single step that removes a carbon dioxide group from the head group (Zborowski et al. 1983). Disruption of the PSD pathway is lethal in mice and causes mitochondrial dysfunction (Steenbergen et al. 2005). Here, the rate-limiting step lies in the ATP-dependent translocation of PS from the ER to the site of PSD at the inner mitochondrial membrane.
Interestingly, ER and mitochondria are not as independent as they seem, and these two pathways can take place in relative proximity thanks to mitochondria-associated ER membranes (MAM). It seems that PSs rather than PEs are exchanged between ER and mitochondria, and PE levels in the mitochondria increase via the PSD pathway rather than by direct exchange with ER membranes (Shiao et al. 1995).
To learn more about the contribution of MAMs to phospholipid transport, watch this webinar by Professor Jean Vance, whose research is behind many of the literature cited in this paragraph.
Another noteworthy observation is that in mammals, while PS decarboxylation yields PEs, triple methylation of PEs by the enzyme phosphatidylethanolamine N-methyltransferase (PEMT) yields PCs. This reaction is responsible for about a third of PC synthesis in the liver, providing PCs that are secreted in the bile to help digestion of lipids (Vance et al. 2013). In addition, the two branches of the Kennedy pathway meet in several places to convert intermediaries of PE synthesis to their equivalent in the PC branch (van der Veen et al. 2017).
Phosphatidylethanolamines and membranes
In animals, PEs are enriched in inner membranes. The exact ratio of PEs to total membrane lipids or phospholipids varies depending on species, cell type, and organelle, but PCs and PEs usually top the phospholipid list. PEs constitute roughly 40% of the phospholipids of inner mitochondrial membranes and 15–25% of the phospholipids in other membranes (van der Veen et al. 2017).
As is the case with many lipid classes, “phosphatidylethanolamine” is often used in singular form, as if there were only one chemical structure for PE. However, as for PCs and other lipids that integrate fatty acyl groups in their structure, PEs are a large group of metabolites. Their structures vary according to the length and number of unsaturations of these fatty acyl groups, which confer a range of properties (Hishikawa et al. 2014).
With their typically conical shape, PEs impose a negative curvature to membranes (McMahon et al. 2015). Their detailed structure is also significant, as it influences membrane fluidity (Dawaliby et al. 2016). Typically, saturated fatty acyl groups will reduce membrane fluidity, while unsaturated groups will increase fluidity. Since the inner mitochondrial membrane is rich in PEs and poor in cholesterol (another lipid classically in charge of regulating membrane fluidity), the exact structure of PEs becomes paramount.
PEs also play a role in cytokinesis and the disassembling of the contractile ring needed for parting cells during cell division (Emoto et al. 2000). In the model organism Trypanosoma brucei (a parasitic protozoa), PEs have been studied for their contribution to cell cycle regulation and their involvement in the assembly of glycosylphosphatidylinositol (GPI) anchors of surface proteins (Signorell et al. 2009; Menon et al. 1993).
For more information on the many functions already described for PEs, see the review by Calzada et al. (2016) where the contribution of PEs to these and other mechanisms are detailed.
Phosphatidylethanolamines, lipoproteins and liver disease
Phospholipids are key components of lipoproteins, the vesicles that transport lipids between organs via the circulation. The lipoproteins most loaded in triglycerides (TGs) are also rich in PEs. These are the chylomicrons that form in intestinal cells to support uptake of dietary lipids, and the very-low-density lipoproteins (VLDLs) that form in hepatocytes to distribute lipids (mainly TGs but also cholesteryl esters) to other organs.
Although PCs constitute the bulk of the phospholipids that form the vesicle, PEs are also present, with functions in vesicle assembly and secretion. If PE levels are too low during assembly of VLDL in liver cells, nascent VLDLs are discarded. In addition, PE levels are shown to decrease with VLDL “age”, pointing to a signal for quick uptake of newly formed vesicle based on their high PE content.
Besides absolute phospholipid concentrations, the PC/PE ratio also attracts interest among researchers. A low PC/PE ratio was observed in liver biopsies and in erythrocytes of patients with non-alcoholic fatty liver disease (NAFLD) (Arendt et al. 2013). In knockout mice lacking PEMT activity (the enzyme responsible for the conversion of PEs to PCs in the liver), a low PC/PE ratio is associated with more permeable hepatocytes membranes, leading to hepatic damage (Li et al. 2006). Interestingly, blocking PE synthesis by blocking the CDP-ethanolamine pathway leads to TG accumulation and liver steatosis (Leonardi et al. 2009).
Phosphatidylethanolamines, neurology and aging
PEs also attract attention as metabolites that change with aging (Pradas et al. 2019; Dai et al. 2021). In the human brain, post-mortem analysis has shown that overall PE levels decreased with age in mitochondrial membranes. For example, the levels of the polyunsaturated fatty acid (PUFA)-containing PE 18:0_22:4 decreased by 20% in the mitochondria of the hippocampus in adults aged between 20 and 100 (Hancock et al. 2015).
Naturally, the discovery of links between PEs and aging have triggered research into the use of PE supplementation to increase lifespan. Positive results have been seen in model organisms and human cells via stimulation of autophagy (Rockenfeller et al. 2015).
PEs are also a point of interest for research into neurodegenerative diseases such as Alzheimer’s and Parkinson’s diseases, specifically in relation to PEs role in mitochondrial functions, including oxidative phosphorylation. Reducing PE synthesis has been shown to increase mitochondrial membrane potential and reduce activity of the electron transport chain and ATP production (Tasseva et al. 2013).
Synthesis of amyloid beta, a prominent peptide in the pathophysiology of Alzheimer’s disease, is synthesized by enzymes anchored in membranes. In cell models and in Drosophila melanogaster (fruit fly), amyloid beta production was found to increase when membrane PE levels were reduced, and decrease when PE levels were increased. (Nesic et al. 2012).
Learn more about the roles of PEs and other phospholipids in complex chronic diseases such as cancer, Alzheimer’s disease, depression, inflammatory bowel disease, multiple sclerosis and diabetes in our whitepaper “Complex chronic diseases have a common origin”.
References
Calzada E. et al.: Phosphatidylethanolamine Metabolism in Health and Disease. (2016) International review of cell and molecular biology | http://doi.org/10.1016/bs.ircmb.2015.10.001
Cohn J. et al.: Dietary phospholipids and intestinal cholesterol absorption. (2010) Nutrients | http://doi.org/10.3390/nu2020116
Dai Y. et al.: The Crucial Roles of Phospholipids in Aging and Lifespan Regulation. (2021) Frontiers in physiology | http://doi.org/10.3389/fphys.2021.775648
Dawaliby R. et al.: Phosphatidylethanolamine Is a Key Regulator of Membrane Fluidity in Eukaryotic Cells. (2016) The Journal of Biological Chemistry | https://doi.org/10.1074/jbc.M115.706523
Emoto K. et al.: An essential role for a membrane lipid in cytokinesis. Regulation of contractile ring disassembly by redistribution of phosphatidylethanolamine. (2000) The Journal of cell biology | https://doi.org/10.1083/jcb.149.6.1215
Hancock S. et al.: Decreases in Phospholipids Containing Adrenic and Arachidonic Acids Occur in the Human Hippocampus over the Adult Lifespan. (2015) Lipids | https://doi.org/10.1007/s11745-015-4030-z
Hishikawa D. et al.: Diversity and function of membrane glycerophospholipids generated by the remodeling pathway in mammalian cells. (2014) Journal of Lipid Research | https://doi.org/10.1194/jlr.R046094
Ikuo I. et al.: Absorption and transport of base moieties of phosphatidylcholine and phosphatidylethanolamine in rats. (1987) Biochimica et Biophysica Acta (BBA) – Lipids and Lipid Metabolism | https://doi.org/10.1016/0005-2760(87)90024-5
McMahon H. et al.: Membrane curvature at a glance. (2015) Journal of Cell Science | https://doi.org/10.1242/jcs.114454
Menon A. et al.: Phosphatidylethanolamine is the donor of the terminal phosphoethanolamine group in trypanosome glycosylphosphatidylinositols. (1993) The EMBO Journal | https://doi.org/10.1002/j.1460-2075.1993.tb05839.x
Nesic I. et al.: Alterations in phosphatidylethanolamine levels affect the generation of Aβ. (2012) Aging cell | https://doi.org/10.1111/j.1474-9726.2011.00760.x
Patel D. et al.: Ethanolamine and Phosphatidylethanolamine: Partners in Health and Disease. (2017) Oxidative Medicine and Cellular Longevity | https://doi.org/10.1155/2017/4829180
Pradas I. et al.: Exceptional human longevity is associated with a specific plasma phenotype of ether lipids. (2019) Redox Biology | https://doi.org/10.1016/j.redox.2019.101127
Rockenfeller P. et al.: Phosphatidylethanolamine positively regulates autophagy and longevity. (2015) Cell death and differentiation | https://doi.org/10.1038/cdd.2014.219
Shiao Y. et al.: Evidence that phosphatidylserine is imported into mitochondria via a mitochondria-associated membrane and that the majority of mitochondrial phosphatidylethanolamine is derived from decarboxylation of phosphatidylserine. (1995) The Journal of Biological Chemistry | https://doi.org/10.1074/jbc.270.19.11190
Signorell A. et al.: Perturbation of phosphatidylethanolamine synthesis affects mitochondrial morphology and cell-cycle progression in procyclic-form Trypanosoma brucei. (2009) Molecular Microbiology | https://doi.org/10.1111/j.1365-2958.2009.06713.x
Steenbergen R. et al.: Disruption of the phosphatidylserine decarboxylase gene in mice causes embryonic lethality and mitochondrial defects. (2005) The Journal of Biological Chemistry | https://doi.org/10.1074/jbc.M506510200
Tasseva G. et al.: Phosphatidylethanolamine deficiency in Mammalian mitochondria impairs oxidative phosphorylation and alters mitochondrial morphology. (2013) The Journal of Biological Chemistry | https://doi.org/10.1074/jbc.M112.434183
van der Veen J. et al.: The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. (2017) Biochimica et Biophysica Acta (BBA) – Biomembranes | https://doi.org/10.1016/j.bbamem.2017.04.006
Vance J. et al.: Formation and function of phosphatidylserine and phosphatidylethanolamine in mammalian cells. (2013) Biochimica et biophysica acta | https://doi.org/10.1016/j.bbalip.2012.08.016
Zborowski J. et al.: Phosphatidylserine decarboxylase is located on the external side of the inner mitochondrial membrane. (1983) FEBS Letters | https://doi.org/10.1016/0014-5793(83)81141-7