- History & evolution
- Biosynthesis vs. dietary uptake
- Circulating cholesterol and cardiovascular disease
- Cholesterol as a precursor
- Cholesterol and membranes
- Cholesterol and neurology
- Cholesterol and cancer
History and evolution
1769: first discovery (Olson 1998) | 1929: discovery of lipoproteins | 1951: first synthesis of cholesterol
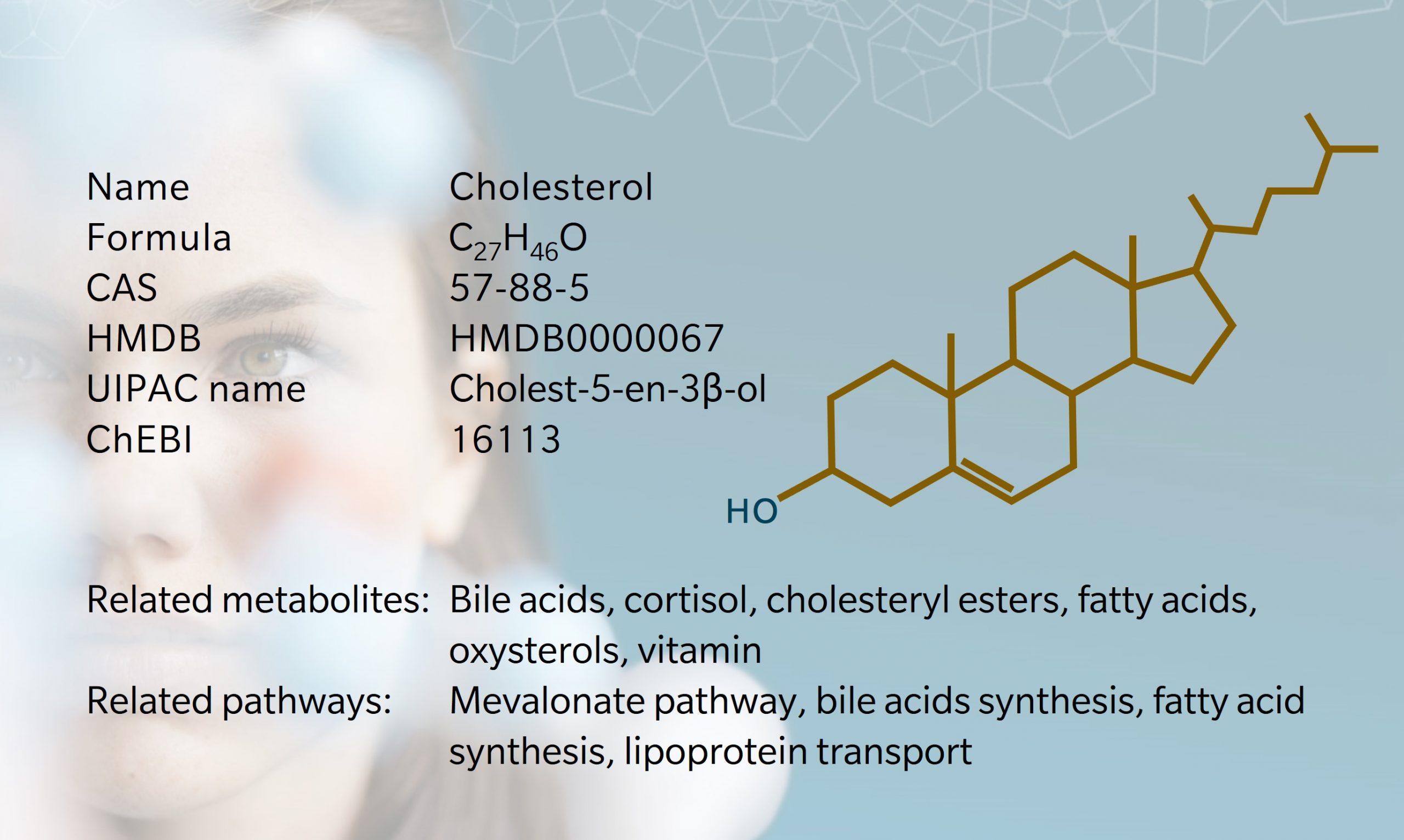
Cholesterol is a lipid found in all animal cell membranes, contributing to cell structure and integrity, membrane fluidity, and transportation of signaling molecules (Goldstein et al. 2009). It is also a precursor to steroid hormones, bile acids and vitamin D.
The study of cholesterol has a long history. It was first discovered in 1769 by Poulletier de la Salle, who observed a waxy substance in bile and gallstones (Olson 1998). The name is derived from “cholesterine”, a name given to it by Chevreul in 1815 based on the Ancient Greek words for bile (chole) and solid (stereos). By the early 19th century, cholesterol was becoming better understood as a component of blood plasma.
Cholesterol lipoproteins were discovered in 1929, with the first substance isolated later shown to be high-density lipoprotein (HDL) (Olson 1998). The second of the two main cholesterol-carrying lipoproteins, low-density lipoprotein (LDL), was discovered in 1949, when John Gofman took advantage of the Manhattan Project’s ultracentrifuge technology to investigate “protein X” (LDL) (Siri-Tarino et al. 2006).
In the 1960s and 70s, the protein components and receptors of cholesterol lipoproteins were identified, allowing a greater understanding of its role in the fat transport system. This led to the “cholesterol wars”, where controversy surrounding the role of lipids (specifically dietary cholesterol) in atherosclerosis and coronary heart disease dominated lipid research for the remainder of the 20th century, arguably hindering medical progress (Kuijpers 2021).
Biosynthesis vs. dietary uptake
Around 70% of cholesterol in humans is synthesized endogenously, with the remaining 30% obtained via the diet (Bourgin et al 2020). Biosynthesis of cholesterol occurs in the liver through either de novo or exogenous biosynthesis. De novo biosynthesis is a complex process, starting with acetyl-coenzyme A (CoA), derived via oxidation reactions (Tsuchiya et al. 2010). Acetyl-CoA condenses to 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA). This is catalyzed by HMG-CoA synthase and reduced by HMG-CoA reductase to produce mevalonate. Mevalonate is then converted to squalene, a precursor of sterols, via a series of enzymatic reactions. A further phase of enzymatic reactions converts these sterols into cholesterol in the endoplasmic reticulum.
In the exogenous pathway, dietary cholesterol comes from steroids in animal tissues, with the main sources including eggs, meat and dairy products. To be absorbed, ingested cholesterol esters must be hydrolyzed by pancreatic enzymes to form free fatty acids (FFAs) (Lecerf 2011). Around 1000 mg of cholesterol reaches the intestinal lumen each day, from both endogenous and exogenous pathways.
Cholesterol homeostasis is maintained via feedback loops regulated by transcriptional factors, liver X receptors (LXR, which regulate cholesterol excretion) and farnesoid X receptors (FXR, which regulate bile acid metabolism) (Soliman 2018).
Circulating cholesterol and cardiovascular disease
Cholesterol is excreted from the liver into biliary fluids, which then enter the digestive system. Around half of this cholesterol is recycled via the small intestine (Cohn 2010). HDL helps transport excess fat from the tissues and blood to the liver, where it can be excreted from the body. LDL does the opposite, transporting fat to the tissues.
Cholesterol gets mixed reviews, with HDL often labelled as “good” cholesterol and LDL considered “bad”. Elevated HDL may have a protective effect, and too little can increase the risk of cardiovascular disease. Conversely, elevated LDL levels can cause a build-up of fats in the blood, causing clots and cardiovascular disease (Soliman 2018). However, both play an essential role in human health, and an imbalance of either can have deleterious effects, so it is now commonly accepted that the names “good” and “bad” cholesterol make little sense.
Cholesterol levels in extracellular vesicles (EVs) have attracted attention as potential diagnostic markers of cardiovascular disease, cancer and other diseases (Pfrieger et al. 2018). EVs contain cholesterol metabolites such as 27-hydroxycholesterol, which has been associated with breast cancer cell lines (Roberg-Larsen 2017). These membrane-enclosed structures released by cells may also be a method of drug delivery, by transporting proteins, genes, microRNAs and viruses to treat disease (Pfrieger et al. 2018).
Cholesterol as a precursor
Cholesterol is a precursor of the following metabolites:
• bile acids (such as cholic acid), which aid digestion, contribute to the absorption of lipophilic nutrients in the intestine, and support gene and metabolism regulation;
• steroid hormones (such as cortisol and sex hormones) which regulate numerous physiological functions and metabolic processes, and are synthesized from cholesterol in the adrenal gland and gonads through the common precursor steroid pregnenolone, using the LDL/LDL-receptor pathway (Hu et al. 2010);
• vitamin D, which helps regulate calcium and phosphate levels and supports bone, dental, muscular and paracrine functions;
• oxysterols, which are oxygenated derivatives of cholesterol that play a role in bile acid synthesis, the immune system and bone marrow function (Oguro 2019).
Cholesterol and membranes
Cholesterol is an essential building block of cell membranes, interacting with phospholipid fatty-acid chains to influence membrane fluidity and integrity. It does this primarily through sphingolipid rafts. Lipid rafts help regulate cell signaling, membrane transport and protein trafficking, though precisely how they affect cholesterol transport is still unknown (Cerqueira et al. 2016, Wang et al. 2020). The involvement of cholesterol-enriched lipid rafts has been observed in studies of viruses including influenza, human immunodeficiency virus (HIV), Newcastle disease virus (NDV), herpes and others.
Cholesterol is an important component of the myelin sheath, along with sphingomyelins (Poitelon et al. 2020). The myelin sheath surrounds nerve cell axons, which makes it relevant to the study of diseases related to the central nervous system and neurophysiology.
Myelin cannot be synthesized without cholesterol and contains the largest pool of free cholesterol in the body (Poitelonet al. 2020). Most cholesterol in myelin is derived from de novo synthesis in oligodendrocytes and astrocytes, since blood-based cholesterol cannot cross the blood-brain barrier.
Cholesterol and neurology
Around a quarter of the body’s cholesterol is contained in the brain (Björkhem et al. 2004). As noted, cholesterol availability is a crucial factor in brain development and neuronal function. Changes in cholesterol metabolism in the central nervous system have been implicated in neurologic disorders, including Alzheimer’s disease (AD), Huntington’s disease and Parkinson’s disease (Zhang et al. 2015).
Several studies have linked hypercholesterinemia and lipoprotein isoforms such as ApoE4 to AD. One study showed that lower levels of cholesterol precursors may reduce the neuroprotective effect on mitochondrial energy metabolism, while non-enzymatically produced metabolites may contribute to inflammation, pointing to cholesterol metabolism as a potential therapeutic target in AD (Varma et al. 2021).
Smith-Lemli-Opitz syndrome is a rare recessive disorder caused by mutations in the gene encoding enzyme 7-dehydrocholesterol reductase. Patients with severe cases can experience intellectual disabilities, delayed motor and language development, and sleep disorders, thought to be exacerbated by low brain cholesterol levels (Cunniff et al. 1997).
Cholesterol and cancer
Cholesterol has a known impact on carcinogenic activity, including tumor development, apoptosis, and angiogenesis (Cruz et al. 2013). Several studies have investigated cholesterol metabolism as a potential therapeutic target for cancer treatment. Inhibiting cholesterol synthesis via the mevalonate pathway and delivering anti-cancer agents via lipoprotein transport are two examples.
One study used the drug ezetimibe, in combination with dietary interventions, to show that elevated circulating cholesterol levels promote prostate cancer tumor growth (Solomon et al. 2009). Reduced circulating cholesterol was shown to slow tumor growth.
Other studies have taken a “Trojan horse” approach to exploit cancer cells’ high cholesterol requirements, to deliver anti-cancer agents to cancer cells (Cruz et al. 2013). Further research is needed to understand more about these different strategies.
References
Björkhem I. et al.: Brain Cholesterol: Long Secret Life Behind a Barrier. (2004) Arteriosclerosis, Thrombosis, and Vascular Biology | https://doi.org/10.1161/01.ATV.0000120374.59826.1b
Bourgin M. et al.: Exploring the Bacterial Impact on Cholesterol Cycle: A Numerical Study. (2020) Frontiers in Microbiology | https://doi.org/10.3389/fmicb.2020.01121
Cerqueira N. et al.: Cholesterol Biosynthesis: A Mechanistic Overview. (2016) Biochemistry | https://doi.org/10.1021/acs.biochem.6b00342
Cohn J. et al.: Dietary Phospholipids and Intestinal Cholesterol Absorption. (2010) Nutrients | https://doi.org/10.3390/nu2020116
Cruz P. et al. : The role of cholesterol metabolism and cholesterol transport in carcinogenesis: a review of scientific findings, relevant to future cancer therapeutics.” ( 2013) Frontiers in Pharmacology | https://doi.org/10.3389/fphar.2013.00119
Cunniff C. et al.: Clinical and biochemical spectrum of patients with RSH/Smith-Lemli-Opitz syndrome and abnormal cholesterol metabolism. (1997) American Journal of Medical Genetics | https://doi.org/10.1007/978-1-4614-1037-9_218
Goldstein J. et al.: History of Discovery: The LDL Receptor. (2009) Arterioscler Thromb Vasc Biol | https://doi.org/10.1161/ATVBAHA.108.179564
Hu J. et al.: Cellular cholesterol delivery, intracellular processing and utilization for biosynthesis of steroid hormones.” (2010) Nutrition & Metabolism | https://doi.org/10.1186/1743-7075-7-47
Kuijpers P.: History in medicine: the story of cholesterol, lipids and cardiology.” (2021) e-Journal of Cardiology Practice | https://www.escardio.org/Journals/E-Journal-of-Cardiology-Practice/Volume-19/history-in-medicine-the-story-of-cholesterol-lipids-and-cardiology]
Lecerf J. et al.: Dietary cholesterol: from physiology to cardiovascular risk. (2011) British Journal of Nutrition | https://doi.org/10.1017/S0007114511000237
Oguro H.: The Roles of Cholesterol and Its Metabolites in Normal and Malignant Hematopoiesis. (2019) Frontiers in Endocrinology | https://doi.org/10.3389/fendo.2019.00204
Olson R.: Discovery of the Lipoproteins, Their Role in Fat Transport and Their Significance as Risk Factors. (1998) The Journal of Nutrition | https://doi.org/10.1093/jn/128.2.439S
Pfrieger F.: Cholesterol and the journey of extracellular vesicles. (2018) Journal of Lipid Research | https://doi.org/10.1194/jlr.R084210
Poitelon Y. et al.: Myelin Fat Facts: An Overview of Lipids and Fatty Acid Metabolism. (2020) Cells | https://doi.org/10.3390/cells9040812
Roberg-Larsen H. et al.: Mass spectrometric detection of 27-hydroxycholesterol in breast cancer exosomes. (2017) The Journal of Steroid Biochemistry and Molecular Biology | https://doi.org/10.1016/j.jsbmb.2016.02.006
Siri-Tarino P.: The early years of lipoprotein research: from discovery to clinical application. (2006) Journal of Lipid Research | https://doi.org/10.1194/jlr.R069575
Soliman G.: Dietary Cholesterol and the Lack of Evidence in Cardiovascular Disease. (2018) Nutrients | https://doi.org/10.3390/nu10060780
Solomon K. et al.: Ezetimibe Is an Inhibitor of Tumor Angiogenesis. (2009) The American Journal of Pathology | https://doi.org/10.2353/ajpath.2009.080551
Tsuchiya M. et al.: Cholesterol Biosynthesis Pathway Intermediates and Inhibitors Regulate Glucose-Stimulated Insulin Secretion and Secretory Granule Formation in Pancreatic β-Cells. (2010) Endocrinology | https://doi.org/10.1210/en.2010-0623
Varma V. et al.: Abnormal brain cholesterol homeostasis in Alzheimer’s disease—a targeted metabolomic and transcriptomic study. (2021) npj Aging and Mechanisms of Disease | https://doi.org/10.1038/s41514-021-00064-9
Wang Y. et al.: Cholesterol-Rich Lipid Rafts in the Cellular Membrane Play an Essential Role in Avian Reovirus Replication.” (2020) Frontiers in Microbiology | https://doi.org/10.3389/fmicb.2020.597794
Zhang J.et al.: Cholesterol metabolism and homeostasis in the brain. (2015) Protein Cell | https://doi.org/10.1007/s13238-014-0131-3