History & evolution
Biosynthesis vs. dietary uptake
S1P signaling
S1P and metabolic disease
S1P and cancer
S1P and neurology
S1P and asthma
History and evolution
1968: discovery (Stoffel et al. 1968) | 1992: first synthesis (Boumendjel and Miller 1994) | 1997: identification of S1P receptors (An et al. 1997)
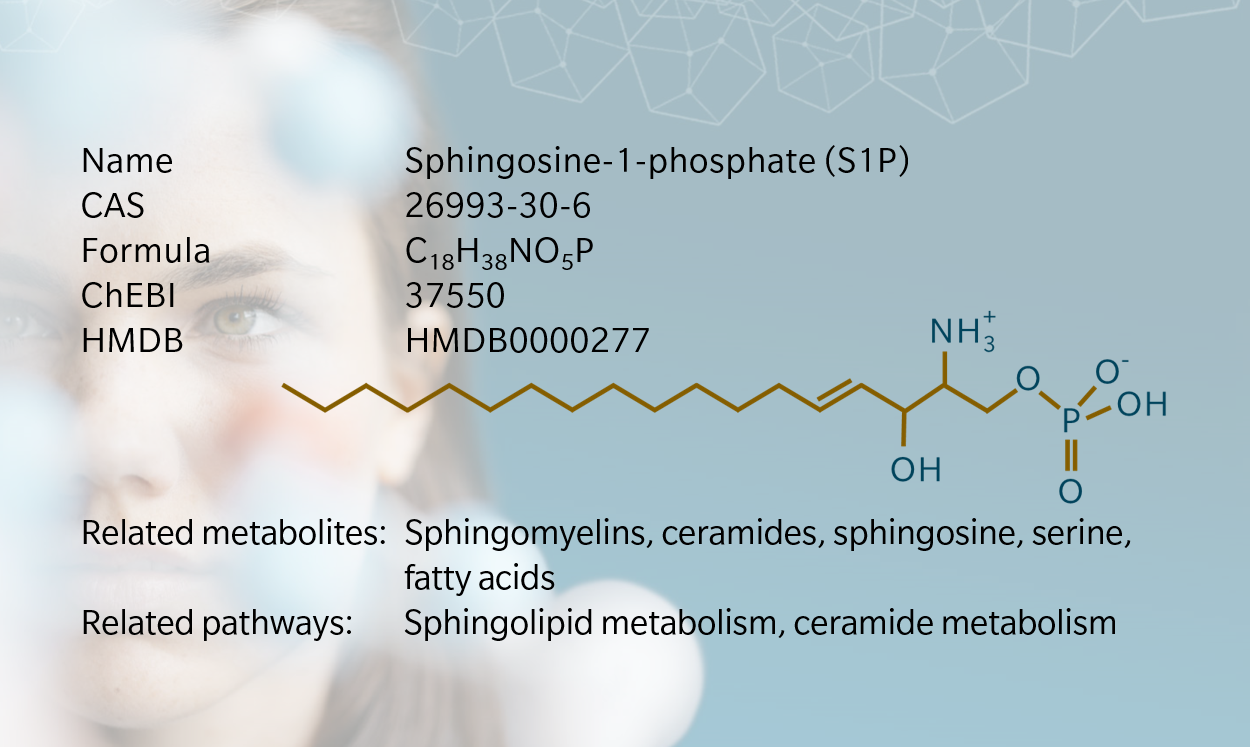
Sphingolipids are the building blocks of cell membranes, first discovered by Thudichum in 1874 (Chatzikonstantinou et al. 2021). Sphingosine-1 phosphate (S1P) was identified as a downstream product of sphingolipid metabolism in the 1960s (Stoffel et al. 1968), and since then has had a significant impact on our understanding of the role of sphingolipids in health and disease.
S1P is a bioactive signaling lipid involved in many cellular processes including cell proliferation, differentiation, inflammation and degradation (Strub et al. 2010). Together with ceramides and sphingosine, S1P forms part of the “sphingolipid rheostat”, which determines cell fate and has become a focus in oncology research (Spiegel and Milstien 2003) (Newton et al. 2015).
Scientists originally attributed S1P’s involvement in cellular processes to its being an intracellular second messenger. However, by the mid-1990s, S1P was found to bind to G protein-coupled receptors (GPCRs) that influence cell growth, motility and cell proliferation (Mendelson et al. 2014). The discovery of S1P receptors was a major milestone in sphingolipid and cell biology research (Park and Im 2017).
Through intracellular and extracellular signaling, S1P and its receptors regulate physiological processes in the central nervous, cardiovascular and immune systems (Hla and Brinkmann 2011). This makes it a metabolite of interest in a wide range of diseases, including cancer, Alzheimer’s disease (AD), atherosclerosis, diabetes and osteoporosis (Maceyka et al. 2012).
Biosynthesis vs. dietary uptake
S1P is a product of ceramide metabolism and can be produced both endogenously and through dietary uptake. De novo synthesis begins in the endoplasmic reticulum (Cantalupo and Lorenzo 2016). Serine palmitoyltransferase converts serine and palmitoyl coenzyme A to produce a ceramide precursor. Several enzymatic reactions result in ceramide, which is converted to sphingosine. Sphingosine is then phosphorylated by sphingosine kinases 1 and 2 (SPhK1 and SphK2) to form S1P (Chatzikonstantinou et al. 2021). S1P participates in intracellular and extracellular processes, ultimately activating S1P receptors in cells throughout the body (Cantalupo and Lorenzo 2016).
To a lesser extent, S1P can be generated through the diet (Norris and Blesso 2017). Sphingolipids are prevalent in diets containing meat, eggs and dairy. Western diets are estimated to contain 200-400mg of sphingolipids per day, with the majority being sphingomyelins (Norris and Blesso 2017). Gut enzymes break these down into ceramides, which can then be converted to sphingosine and enter the S1P pathway (Norris and Blesso 2017). Sphingolipids absorbed in the gastrointestinal (GI) tract affect gut microbiota and thus play a role in GI barrier function, which can lead to inflammation and inflammatory disease (Chatzikonstantinou et al., 2021).
S1P signaling
S1P signaling affects multiple physiological processes but is particularly important for vascular function and blood flow, neurogenesis, endothelial integrity, lymphocyte distribution and embryogenesis (Proia and Hla 2015).
Extracellular S1P signaling occurs via five GPCRs that comprise the endothelial differentiation gene family (S1PR1-S1PR5) (Adada et al. 2013). The actions of these receptors vary according to cell type and environment (Pérez-Jeldres et al. 2021). As the most ubiquitous, S1PR1 is the most widely researched as a target for therapeutic strategies. Much of this research focuses on the “S1P gradient”, which refers to the fact that S1P concentrations are lower in tissues and higher in blood (Hla et al. 2008). This gradient provides a cue for lymphocyte trafficking, and S1PR1 signaling plays a major role in modulating lymphocytes’ access to inflammation sites. Therefore, the S1P-S1PR1 pathway is of interest to researchers developing drugs for multiple sclerosis (MS), inflammatory bowel disease (IBD), rheumatoid arthritis, systemic lupus erythematosus, psoriasis, and other autoimmune diseases (Pérez-Jeldres et al. 2021).
S1P is also known to act on other intracellular targets independently of these receptors (Strub et al. 2010). It is known to inhibit histone deacetylases (HDACs), activate TNF-receptor associated factor 2, and activate beta-secretase 1 (Maceyka et al. 2012). As a result, S1P is implicated in the pathophysiology of diseases in almost every organ.
S1P and metabolic disease
S1P has been identified as a key player in the development of metabolic disease, including diabetes, non-alcoholic fatty liver disease, hepatic steatosis, IBD and colorectal cancer (Kwong and Zhou 2019). It can have both beneficial and deleterious effects.
S1P has been found to be elevated in humans with obesity, correlating with body mass index, body fat percentage and fasting plasma insulin levels (Kowalski et al. 2013). It is active in the main insulin signaling pathways in the liver, adipose tissue and skeletal muscle, and its involvement in lipolysis, glucose uptake and insulin resistance makes it a prominent target for research into diabetes (Green et al. 2021).
Animal models have shown that the SphK1/S1P axis affects diet-related hepatic inflammation, suggesting that higher levels of circulating S1P could ameliorate the effects of a high fat diet and obesity (Choi and Snider 2015).
The release of S1P from high-density lipoprotein (HDL) has been shown to have a protective effect against atherosclerosis, with mouse models showing that S1P analogues and S1PR2 inhibition lead to reduced atherosclerotic plaque formation (Kwong and Zhou 2019). More research is needed to understand the role of S1P in metabolic disease and to determine whether it is a cause or symptom of metabolic dysregulation.
S1P and cancer
Numerous studies have shown that S1P is involved in tumorigenesis (Pyne and Pyne 2010). It regulates the growth and survival of cancer cells, while its precursor, SphK1, is expressed in many types of cancer and correlates with disease progression (Pyne and Pyne 2010). S1PR1 and S1PR3 have been found to promote tumor development, while S1PR2 can both promote and inhibit cancer (Wang et al. 2019).
Research suggests that S1P signaling is a potential therapeutic target, through a reduction in S1P levels or through S1PR agonists and antagonists (Wang et al. 2019) (Nagahashi et al. 2018). Fingolimod, an S1P agonist originally approved for the treatment of MS, has been shown to inhibit tumor growth in lung cancer, colon cancer, hepatocellular carcinoma and prostate cancer (Wang et al. 2019).
Several studies have shown that activation of S1P signaling pathways is linked to colitis, IBD and colorectal cancer, via its involvement in endothelial barrier function and chronic gut inflammation, which increases the risk of cancer (Kwong and Zhou 2019). This suggests that S1P could be an effective therapeutic target to reduce inflammation and associated cancers. However, research also shows that S1P is not always harmful: it has a protective effect on some tissues, including the heart, brain, lung and kidney (Kwong and Zhou 2019).
A study using metabolomics in combination with transcriptomic and genomic data found that targeting S1P could be a promising therapy for some patients with triple-negative breast cancer (Xiao et al. 2022).
S1P and neurology
There is growing evidence of S1P signaling in neurodegenerative, neuroinflammatory and cerebrovascular diseases and conditions, linked to S1P expression in the central nervous system (CNS) (Lucaciu et al. 2020).
This has led to the development of S1P modulators to alleviate systemic immune responses in diseases such as MS. Four such drugs have regulatory approval for MS, including fingolimod, siponimod, ozanimod and ponesimod (McGinley and Cohen 2021). These drugs work primarily by inhibiting lymphocyte response to the S1P gradient, thus reducing the amount of circulating lymphocytes and limiting the migration of inflammatory cells into the CNS (McGinley and Cohen 2021). A second mechanism of action may involve modulation of the microbiota-gut-brain axis, resulting in stabilization of the intestinal barrier and limiting the chronic inflammation and dysbiosis associated with MS (Chatzikonstantinou et al. 2021). While these drugs are used in clinical practice, there is mixed evidence for their efficacy and more research is needed (Cohen and Chun 2011) (Chitnis et al. 2018).
Sphingolipid metabolism also appears to play an important role in Alzheimer’s disease. Patients with AD have been found to have lower levels of S1P than healthy controls (Yin et al. 2023).
A multi-omics study demonstrated that the sphingomyelin pathway is dysregulated in the brains of AD patients compared to healthy controls (Baloni, Arnold et al. 2022). S1P was shown to regulate this pathway in post-mortem human samples as well as in vivo in mouse models. Metabolite genome-wide association studies (mGWAS) identified S1P as a potential drug target. Fingolimod (the S1P modulator) was shown to improve cognition in amyloidogenic mice.
S1P and asthma
On a recent episode of The Metabolomist, Rachel Kelly, Assistant Professor at the Brigham and Women’s Hospital in Boston, Massachusetts (US) shared that S1P is her favorite metabolite, as a result of a study into childhood asthma (Kelly, Mendez et al. 2021). The study tested the hypothesis that vitamin D supplementation in pregnant women led to a lower risk of asthma in their children. The results showed that the children of women who took vitamin D did have a lower risk of asthma, apart from a subset of children who were not producing enough S1P. Kelly explains:
“In mothers supplemented with vitamin D, the children did have an overall reduced risk of asthma, but it wasn’t the case for every child. And that seemed to relate to these children having a specific genotype where they were not producing enough S1P and sphingolipids. So, it didn’t matter how much vitamin D you put into their system, the levels of protective sphingolipids were not increasing, and their risk of asthma was not affected by vitamin D.”
Kelly notes that S1P has also proven to be a relevant metabolite in autism and mental health conditions, and that interest in S1P is growing across a wide range of other diseases and conditions.
To learn more about the roles of metabolites and the microbiome in the fields of metabolic diseases, cancer, neurology, and autoimmune diseases, download our whitepaper “Complex chronic diseases have a common origin”.
References
Adada et al.: Sphingosine-1-phosphate receptor 2. (2013) FEBS J | https://doi.org/10.1111/febs.12446
An et al.: Identification of cDNAs encoding two G protein-coupled receptors for lysosphingolipids. (1997) FEBS Lett | https://doi.org/10.1016/s0014-5793(97)01301-x
Baloni et al.: Multi-Omic analyses characterize the ceramide/sphingomyelin pathway as a therapeutic target in Alzheimer’s disease. (2022) Commun Biol | https://doi.org/10.1038/s42003-022-04011-6
Boumendjel et al.: Synthesis of sphingosine-1-phosphate and dihydrosphingosine-1-phosphate. (1994) Journal of Lipid Research | https://doi.org/10.1016/S0022-2275(20)39936-3
Cantalupo et al.: S1P Signaling and De Novo Biosynthesis in Blood Pressure Homeostasis. (2016) J Pharmacol Exp Ther | https://doi.org/10.1124/jpet.116.233205
Chatzikonstantinou et al.: Signaling through the S1P−S1PR Axis in the Gut, the Immune and the Central Nervous System in Multiple Sclerosis: Implication for Pathogenesis and Treatment. (2021) Cells | https://doi.org/10.3390/cells10113217
Chitnis et al.: Trial of Fingolimod versus Interferon Beta-1a in Pediatric Multiple Sclerosis. (2018) N Engl J Med | https://doi.org/10.1056/NEJMoa1800149
Choi et al.: Sphingolipids in High Fat Diet and Obesity-Related Diseases. (2015) Mediators Inflamm | https://doi.org/10.1155/2015/520618
Cohen et al.: Mechanisms of fingolimod’s efficacy and adverse effects in multiple sclerosis. (2011) Annals of Neurology | https://doi.org/10.1002/ana.22426
Green et al.: Sphingolipids in Metabolic Disease: The Good, the Bad, and the Unknown. (2021) Cell Metab | https://doi.org/10.1016/j.cmet.2021.06.006
Hla and Brinkmann et al.: Sphingosine 1-phosphate (S1P): Physiology and the effects of S1P receptor modulation. (2011) Neurology | https://doi.org/10.1212/WNL.0b013e31820d5ec1
Hla et al.: The vascular S1P gradient—Cellular sources and biological significance. (2008) Biochim Biophys Acta | https://doi.org/10.1016/j.bbalip.2008.07.003
Kelly et al.: Metabo-Endotypes of Asthma Reveal Differences in Lung Function: Discovery and Validation in Two TOPMed Cohorts. (2021) ATS J | https://doi.org/10.1164/rccm.202105-1268OC
Kowalski et al.: Plasma Sphingosine-1-Phosphate Is Elevated in Obesity. (2013) PLoS One | https://doi.org/10.1371/journal.pone.0072449
Kwong et al.: Sphingosine-1-phosphate signaling and the gut-liver axis in liver diseases. (2019) Liver Res | https://doi.org/10.1016/j.livres.2019.02.003
Lucaciu et al.: The S1P–S1PR Axis in Neurological Disorders—Insights into Current and Future Therapeutic Perspectives. (2020) Cells | https://doi.org/10.3390/cells9061515
Maceyka et al.: Sphingosine-1-phosphate signaling and its role in disease. (2012) Trends Cell Biol | https://doi.org/10.1016/j.tcb.2011.09.003
McGinley et al.: Sphingosine 1-phosphate receptor modulators in multiple sclerosis and other conditions. (2021) The Lancet Therapeutic | https://doi.org/10.1016/S0140-6736(21)00244-0
Nagahashi et al.: The role of sphingosine-1-phosphate in inflammation and cancer progression. (2018) Cancer Sci | https://doi.org/10.1111/cas.1380
Norris et al.: Dietary and Endogenous Sphingolipid Metabolism in Chronic Inflammation. (2017) Nutrients | https://doi.org/10.3390/nu9111180
Park et al.: Sphingosine 1-Phosphate Receptor Modulators and Drug Discovery. (2017) Biomol Ther (Seoul) | https://doi.org/10.4062/biomolther.2016.160
Pérez-Jeldres et al.: Targeting Sphingosine-1-Phosphate Signaling in Immune-Mediated Diseases: Beyond Multiple Sclerosis. (2021) Drugs | https://doi.org/10.1007/s40265-021-01528-8
Proia et al.: Emerging biology of sphingosine-1-phosphate: its role in pathogenesis and therapy. (2015) J Clin Invest | https://doi.org/10.1172/JCI76369
Pyne et al.: Sphingosine 1-phosphate and cancer. (2010) Nature Reviews Cancer | https://doi.org/10.1038/nrc2875
Rohrhofer et al.: The Impact of Dietary Sphingolipids on Intestinal Microbiota and Gastrointestinal Immune Homeostasis. (2021) Frontiers in Immunology | https://doi.org/10.3389/fimmu.2021.635704
Stoffel et al.: Metabolism of sphingosine bases. IX. Degradation in vitro of dihydrospingosine and dihydrospingosine phosphate to palmitaldehyde and ethanolamine phosphate. (1968) Hoppe Seylers Z Physiol Chem | https://doi.org/10.1515/bchm2.1968.349.2.1745
Wang et al.: Roles of sphingosine-1-phosphate signaling in cancer. (2019) Cancer Cell International | https://doi.org/10.1186/s12935-019-1014-8
Xiao et al.: Comprehensive metabolomics expands precision medicine for triple-negative breast cancer. (2022) Cell Res | https://doi.org/10.1038/s41422-022-00614-0
Yin et al.: Status of Metabolomic Measurement for Insights in Alzheimer’s Disease Progression—What Is Missing?. (2023) Int. J. Mol. Sci | https://doi.org/10.3390/ijms24054960