Which animal models are most suitable for COVID-19 studies?
The global impact of the COVID-19 pandemic has prompted collaborative research efforts across the world. Thus far, the bulk of these efforts have been geared towards vaccine development and discovery of therapeutic strategies that mitigate disease severity.
It is clear that SARS-CoV-2 will not merely go away, rather, we will likely be battling this virus for much longer than anticipated. On top of this, many COVID-19 survivors experience long-term complications of the infection, as discussed in a recent post. This not only impacts the quality of life of survivors, it also signifies a threat to the burden on healthcare systems. Until the physiological basis for such complications is clarified, we may continue to see negative impacts on healthcare and the overall economy. Thus, it is crucial to understand the biology of the SARS-CoV-2 pathogen, as well as the disease pathophysiology.
Fortunately, several vaccine and therapeutic candidates are now in the pipeline, some of which are close to market authorization. With this in mind, we anticipate a shift in the focus of COVID-19 research. There is still much to learn about the pathophysiology that determines long-term outcomes, in order to provide treatment options that mitigate these long-term effects. Besides clinical research on this topic, it is imperative that basic research studies on COVID-19 utilize appropriate animal models that best mimic human disease. For more on this, see also the review by Ehaideb et al., 2020.
Here, we discuss the potential of various model systems for COVID-19 research. We especially focus on the convergence with human metabolic control, which would be expected to improve the transferability of findings. This may be particularly useful for studies on the long-term complications in survivors where clinical research is complicated, increasing the necessity to resort to studies in model systems.
Compared to proteins, metabolites have been considerably more conserved in evolution. Metabolites are generally identical across species and most metabolic pathways are identical. This makes metabolomics well-suited for translational research, especially for emerging infectious diseases, as fast and efficient transfer of results is of utmost importance. Nonetheless, not all animal models reflect human metabolism to the same extent. Thus, we review here specific areas of metabolism that may affect model suitability.
SARS-CoV-2 preclinical models
- In vitro cell lines.
Though not considered an animal model, cell lines are key for understanding SARS-CoV-2 biology and for early evaluation of therapeutic candidates. As cell lines are derived from humans, they mimic the physiology of human cells. Yet, it is worth noting that differences in glucose utilization have been observed between in vitro activated T-cells compared to T-cells activated physiologically via infection. T-cells activated by infection show greater rates of oxidative metabolism and carbon flux to anabolic pathways, such as synthesis of serine and nucleotides (Ma et al., 2019). Further, certain metabolites are not universally produced by all cell types, so cell-based assays do not fully reflect metabolic interactions between cell types, tissues and organs.
Though SARS-CoV-2 can replicate in the animal models discussed below, studies have also revealed that infection elicits only mild disease in these animals, apart from infrequent exceptions (Ehaideb et al., 2020).
- Small Animals (Hamsters and Mice).
Mice and rats are generally regarded as the go-to animal models for a variety of preclinical studies, due to their size, short life cycles, low costs for upkeep and the availability of tools for genetic manipulation.
It is well established that SARS-CoV-2 enters cells by way of the ACE2 enzyme. Interestingly, it was discovered that the virus is unable to enter cells through mouse ACE2. Thus, researchers now utilize transgenic mice that express human ACE2. However, even if the virus can enter the cells of hACE2 transgenic mice, it is likely that there still may not be full transferability to human studies, due to differences in corticosteroid hormone homeostasis between humans and rodents (Joels et al., 2018). Along with ACE2, these hormones are involved in the Renin-Angiotensin-Aldosterone System (RAAS).
Hamsters have emerged as an excellent replacement for mice in COVID-19 studies. The ACE2 enzyme in hamsters has homology to human ACE2 and molecular docking has suggested sufficient binding between hamster ACE2 and the SARS-CoV-2 S protein. Hamsters were found to exhibit the most consistency in terms of lung disease. Yet, fewer tools are available for hamster studies as they are less commonly used compared to other small animals (Khoury et al., 2020).
- Large Animals (Ferrets and Cats).
These animal models are best suited for studies in which the pathology of COVID-19 in humans does not need to be exactly replicated, but its physiological impact must be assessed. SARS-CoV-2 is capable of replicating in ferrets and cats, though ferrets were found to exhibit greater disease severity. It is worth noting that transmission of a SARS-CoV-2 variant from minks to humans was recently reported in Denmark. The variant contained mutations in the S protein that rendered it less susceptible to neutralizing antibodies. While vaccine or therapeutic candidates are not expected to be any less effective against this variant, further investigation is needed to verify this (ECDC). As both minks and ferrets belong to the Mustelidae family, studies in ferrets may be valuable for these efforts.
- Non-Human Primates.
The pathology of COVID-19 in humans is most closely mimicked by that seen in primates. Though cynomolgus and rhesus macaques typically experience mild disease, they do exhibit some pathologies seen in humans and cases of lethal lung disease have been reported (Khoury et al., 2020). Unsurprisingly, primates have the best physiological similarity to humans (~99% genetic similarity) compared to other animal models, due to their proximity within the evolutionary process. Of course, these models have limitations, including high maintenance costs, long gestation periods and lifetimes.
Metabolic differences between humans and animal models
It is well-acknowledged that studies in animal models are not always fully transferable to humans, and differences in metabolic control may contribute to this effect despite high degrees of evolutionary conservation. In the following section, we discuss possible influences on the transferability of animal model based COVID-19 research, apart from the immediate capability of SARS-CoV-2 to infect those animals and the ACE2 homology described above.
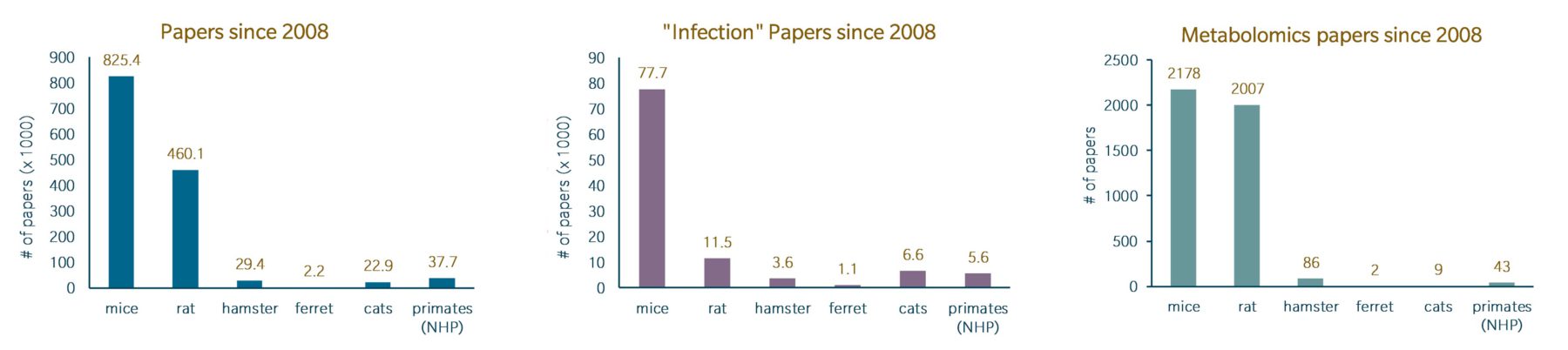
The advantages associated with using rodents in animal studies are reflected in the sheer number of publications using mice and rats compared to other animal models. Their widespread use in a variety of studies has resulted in the identification of metabolic differences that occur in these mammals compared to humans. On the other hand, less is known about the metabolic differences between humans and the other animal models discussed here. However, they certainly may exist. Thus, one should be cognizant of such differences when selecting an animal model for a research project.
Rodents exhibit differences in ascorbate synthesis, purine degradation, and glycan metabolism, as well as hepatocyte growth, compared to humans (Blais et al 2017). Further, prior work has demonstrated notable differences in the adaptive and innate immune systems of mice and humans, as well as in their responses to inflammatory conditions (Mrochen et al, 2020; Akhtar, 2015), which may be reflected in immune modulatory metabolic pathways. Rodents also exhibit differences in lipid metabolism. For example, mice adipocytes exhibit marked differences in the receptor types that control lipid catabolism, and this corresponds to differences in the elicited metabolic responses (Bergen and Mersmann, 2005).
Humanized mouse models of atherosclerosis require significant alterations. While circulating cholesterol is transported via LDL in humans and other mammals, in mice it is transported via HDL. As a result, mice have much lower cholesterol levels, which confers protection against atherosclerosis. As HDL and LDL differ in phospholipid composition, discrepancies may exist between humans and mice in certain phospholipid levels. This can be mimicked in humanized mouse models. Still, as several mouse models of atherosclerosis exist, phospholipid signatures can vary depending on the manipulations used to generate the model (Saulnier-Blache et al, 2018). Further, mice exhibit low absorption of dietary cholesterol and lack a homolog of a human protein that transfers cholesterol esters and triglycerides between lipoproteins (von Scheidt et al., 2017). Further discrepancies in cholesterol/oxysterol metabolism have also been reported (Kilk 2019). Such differences may impact the apparent association between metabolic disease and COVID-19 disease course that have been discussed in previous articles of this series
Rodents can modify bile acids in different ways than humans. For example, a number of hydroxylated bile acids, the so-called muricholic acids, typically make up a large proportion of serum bile acids in rodents but are hardly found in many other species. In addition, in the rodent liver, secondary bile acids can be converted back into primary bile acids – a reaction that does not occur in the human liver. For that reason, studies on bile acid metabolism in these models may not be truly reflective of the processes occurring in humans. (Wishart 2019).
Differences in bile acid metabolism compared to humans are not only known for rodents. Cats also differ in their metabolism of bile acids, as they cannot conjugate bile acids with glycine, only taurine (Hickman, 1992). Cats also differ in the preferred site and carbon source for de novo lipogenesis (DNL). Whereas in humans and rodents, DNL primarily occurs in the liver, with glucose as the preferred carbon source, cats prefer acetate, with DNL mainly occurring in adipose tissue. In cats, the use of acetate obviates the need for carbon flow to pass through the mitochondria prior to cytosolic fatty acid synthesis, thus eliminating the role of certain enzymes involved in regulating lipid metabolism (Bergen and Mersmann, 2005).
Other considerations
Of course, animal models also differ greatly in their gut microbiomes. Given the important functions of the microbiome in shaping both metabolism and immunity, this is an important consideration.
Animal diets should also be well-considered. For example, metabolic diseases that pre-dispose patients to a severe course of COVID-19 often occur against a background of adverse nutritional habits (“Western diet”). High-fat diet is frequent in research on metabolic diseases and related disorders, such as obesity. However, the terms “Western diet” or “High-Fat diet” are poorly defined. Moreover, dietary intervention studies have shown that obesity and adiposity phenotypes are less dramatic in mice fed a high fat diet at too early of an age (Kleinert et al., 2018). Given that lipid metabolism is heavily discussed in relation to the pro- and anti-inflammatory actions of different lipids, the composition and timing of diets in experimental systems should be considered, at least as potential confounders.
Finally, it is recommended that animal model-based research not rely on a single experimental setting. In the authors’ opinion, diversification of experimental procedures serves to boost the validity of results. For example, Pandey et al. (2014) showed that various mouse strains differ in their metabolic reaction to 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) induced steatohepatitis. In using three different mouse strains, robustly perturbed pathways could be differentiated from strain-specific reactions. In addition, Pann et al. (2020) have shown that the metabolic dynamics in early life differs between different mouse strains.
Summary
Preclinical studies that use animal models are undeniably valuable and obligatory to ensure safety and efficacy of therapeutics prior to clinical evaluation. At the same time, there are limitations associated with the use of animal models in mimicking human disease and treatment response. It is unlikely that such challenges, stemming from metabolic differences between species, will ever be fully resolved. Still, conscientious efforts to humanize animal models, based on known metabolic divergences from humans, can aid in the translatability of results and ultimately elucidate unknown aspects of pathophysiology.
Metabolomics represents a means to circumvent some of these challenges. Comparative metabolomics can shed light on metabolic processes that differ between species. This can reveal aspects of a study that researchers should be cautious of when advancing a drug to the clinic. Importantly, the non-invasive sampling approach and minimal sample volume requirements allow for more frequent sample collection. This can offer a comprehensive picture of the mechanisms governing pathophysiological changes in animals and humans. Moreover, such deep insights can rapidly advance our understanding of the long-term complications seen in patients. In particular, targeted metabolomics is highly valuable in COVID-19 studies, as it allows separate groups to pool their data and draw interpretations from separate studies.
References
Akhtar A: The flaws and human harms of animal experimentation (2015) Camb Q Healthc Ethics | https://doi.org/10.1017/S0963180115000079
Bergen WG, Mersmann HJ: Comparative aspects of lipid metabolism: impact on contemporary research and use of animal models (2005) J Nutr. | https://doi.org/10.1093/jn/135.11.2499
Blais, E., Rawls, K., Dougherty, B. et al. Reconciled rat and human metabolic networks for comparative toxicogenomics and biomarker predictions (2017) Nat Commun | https://doi.org/10.1038/ncomms14250
Ehaideb SN, Abdullah ML, Abuyassin B, Bouchama A: Evidence of a wide gap between COVID-19 in humans and animal models: a systematic review. (2020) Crit Care | https://doi.org/10.1186/s13054-020-03304-8
European Centre for Disease Prevention and Control. Detection of new SARS-CoV-2 variants related to mink. Rapid risk assessment. https://www.ecdc.europa.eu/sites/default/files/documents/RRA-SARS-CoV-2-in-mink-12-nov-2020.pdf (ECDC, accessed 24 November 2020).
Hickman MA, Morris JG, Rogers, QR: Intestinal Taurine and the Enterohepatic Circulation of Taurocholic Acid in the Cat. In: Lombardini J.B., Schaffer S.W., Azuma J. (eds) Taurine. Advances in Experimental Medicine and Biology, vol 315. Springer, Boston, MA. https://doi.org/10.1007/978-1-4615-3436-5_6
Joëls M, Karst H, & Sarabdjitsingh RA: The stressed brain of humans and rodents. (2018) Acta physiologica | https://doi.org/10.1111/apha.13066
Khoury DS, Wheatley AK, Ramuta MD, et al.: Measuring immunity to SARS-CoV-2 infection: comparing assays and animal models [published online ahead of print, 2020 Nov 2]. (2020) Nat Rev Immunol. | https://doi.org/10.1038/s41577-020-00471-1
Kilk K. Metabolomics for Animal Models of Rare Human Diseases: An Expert Review and Lessons Learned. OMICS. 2019 Jun;23(6):300-307. doi: 10.1089/omi.2019.0065. Epub 2019 May 23. PMID: 31120384.
Kleinert M, Clemmensen C, Hofmann S et al.: Animal models of obesity and diabetes mellitus. (2018) Nat Rev Endocrinol | https://doi.org/10.1038/nrendo.2017.161
Ma EH, Verway MJ, Johnson RM, et al: Metabolic Profiling Using Stable Isotope Tracing Reveals Distinct Patterns of Glucose Utilization by Physiologically Activated CD8+ T Cells. (2019) Immunity | https://doi.org/10.1016/j.immuni.2019.09.003
Mrochen DM, Fernandes de Oliveira LM, Raafat D, Holtfreter S: Staphylococcus aureus Host Tropism and Its Implications for Murine Infection Models. (2020) Int J Mol Sci | https://doi.org/10.3390/ijms21197061
Pandey, V., Sultan, M., Kashofer, K., Ralser, M., Amstislavskiy, V., Starmann, J., Osprian, I., Grimm, C., Hache, H., Yaspo, M. L., Sültmann, H., Trauner, M., Denk, H., Zatloukal, K., Lehrach, H., & Wierling, C. (2014). Comparative analysis and modeling of the severity of steatohepatitis in DDC-treated mouse strains. PloS one, 9(10), e111006. https://doi.org/10.1371/journal.pone.0111006
Pann P, de Angelis MH, Prehn C, Adamski J: Mouse Age Matters: How Age Affects the Murine Plasma Metabolome (2020) Metabolites | https://doi.org/10.3390/metabo10110472
Saulnier-Blache JS, Wilson R, Klavins K, Graham D, Alesutan I, Kastenmüller G et al.: Ldlr-/- and ApoE-/- mice better mimic the human metabolite signature of increased carotid intima media thickness compared to other animal models of cardiovascular disease. (2018) Atherosclerosis | https://doi.org/10.1016/j.atherosclerosis.2018.07.024
Takayama K: In vitro and Animal Models for SARS-CoV-2 research. (2020) Trends in Pharmacological Sciences | https://doi.org/10.1016/j.tips.2020.05.005
von Scheidt M, Zhao Y, Kurt Z, Pan C, Zeng L, Yang X, Schunkert H, Lusis AJ: Applications and Limitations of Mouse Models for Understanding Human Atherosclerosis. (2017) Cell Metab | https://doi.org/10.1016/j.cmet.2016.11.001
Wishart DS: Metabolomics for Investigating Physiological and Pathophysiological Processes. (2019) Physiol Rev | https://doi.org/10.1152/physrev.00035.2018