- History & Evolution
- Biosynthesis and dietary uptake
- Choline and the gut microbiome
- Choline and cardiovascular diseases
- Choline and neurology
- Choline supplementation and Alzheimer’s disease
- Choline as biomarker
- Potential dietary and microbiome-based interventions with choline
- References
History & Evolution
1849: first isolation | (Zeisel et al., 2012)1862: named after the Greek word for bile | 1930s: first beneficial properties discovered
Choline is an essential nutrient that plays a key role in phospholipid synthesis, with far-reaching effects in human health and disease.
It was first isolated from pig bile in 1849 by Adolph Strecker, who later named it after the Greek word for bile, chole (Zeisel et al., 2012). In these early years, choline was “discovered” multiple times, only for researchers to realize later that they were looking at the same compound: Babo and Hirschbrunn named their new substance “sinkaline” (Griffith et al., 1954), while Oscar Liebreich identified a molecule called “neurine”, a component of what he referred to as “protagon, the mother substance of all” (Zeisel et al., 2012). Investigations into the structure of choline by Adolf von Baeyer in 1867 eventually revealed that choline, sinkaline and neurine were the same molecule (Griffith et al.,1954). (What we now refer to as neurine is a downstream metabolite of choline).
Choline emerged as a potential nutrient in the 1930s, when it was found to prevent fatty liver in dogs and rats (Zeisel et al., 2012). Throughout the 1950s and 60s, researchers began to describe the primary pathways for choline synthesis. By the 1990s, it was established as an essential B vitamin-like nutrient for human health, with food agencies including it in dietary recommendations.
Today, choline’s vital role in cell structure and signaling, neurotransmission, gene expression and lipid metabolism opens fascinating avenues of research spanning a wide range of metabolic processes and pathologies (National Institutes of Health 2022).
Biosynthesis vs. dietary uptake

Humans produce some choline endogenously, but not enough to fulfil its role (Wallace et al. 2018). This means dietary intake must compensate for the deficiency.
De novo synthesis of choline occurs via the phosphatidylethanolamine N-methyltransferase (PEMT) pathway. This process takes place in the liver: a PEMT enzyme methylates phosphatidylethanolamine (PE) through three sequential reactions to form phosphatidylcholine (PC) (Vance et al., 2014). This is the primarily endogenous pathway to generate new choline molecules, though choline can also be obtained through the hydrolyzation of PCs and from the breakdown of phosphocholine to cytidine diphosphate-choline (CDP-choline) (Li et al. 2023).
Dietary choline is found in meat, poultry, fish, dairy products and eggs, as well as cruciferous vegetables and legumes (Fischer et al. 2010). When these foods are consumed, pancreatic and mucosal enzymes facilitate the release of choline, which is absorbed in the small intestine and transported through the portal circulation to the liver. Here, choline is stored along with endogenously derived choline, and further released into the body via the bloodstream and lymphatic circulation.(National Institutes of Health 2022).
Recommended daily intake of choline for men and women is around 550mg and 425mg respectively (Wallace et al. 2018). Age, environment and genetics influence choline requirements (Sanders et al., 2007). Because PEMT enzyme is induced by estrogen, postmenopausal women tend to be more susceptible to choline deficiency than other groups (Fischer et al. 2010).
Choline metabolism produces several important metabolites, thus choline deficiency can affect multiple physiological systems. Acetylcholine, an ester of choline and acetic acid, supports neurotransmission and affects cognitive and motor functions (Leermakers et al., 2015). Betaine, another derivative of choline, is involved in homocysteine synthesis and influences cholesterol levels (Leermakers et al., 2015). Choline is also a precursor of membrane phospholipids such as PC and sphingomyelin, which are critical for cell structure, signaling and transport (Sanders et al., 2007). As a precursor of very-low-density-lipoproteins, PC plays a role in transporting triglycerides from the liver, which may explain links between choline deficiency and hepatosteatosis (Yao et al.,1988; Laura K et al., 2012).
Choline and the gut microbiome
Choline metabolism by gut bacteria in the intestine results in trimethylamine (TMA), which is converted to trimethylamine-N-oxide (TMAO) in the liver (Arias et al., 2020). High levels of TMA can cause trimethylaminuria, characterized by a fish-like body odor (Wallace et al., 2018). There has been speculation that elevated TMAO levels may be linked to atherosclerosis, although the evidence remains inconclusive.
Less than 1% of gut microorganisms contribute to TMA production, but the small amount that do are highly effective (Arias et al., 2020). Several intestinal microbiota are involved in TMA synthesis from choline, including Anaerococcus hydrogenalis, Clostridium asparagiformis, Clostridium hathewayi, Clostridium sporogenes, Desulfovibrio desulfuricans, Escherichia fergusoni, Ed. tarda, Klebsiella pneumoniae, Proteus penneri, and Providencia rettgeri. Levels of TMA and TMAO are particularly influenced by Firmicutes and Proteobacteria populations (Arias et al., 2020).
In mice, low levels of TMA-producing bacteria have been found to significantly reduce choline availability to the host, with more pronounced effects as these bacteria become more abundant (Romano et al., 2015). In humans, dietary choline depletion has been shown to alter Gammaproteobacteria and Erysipelotrichi populations, with a direct effect on levels of liver fat (Spencer et al., 2010).
Choline and cardiovascular disease
There is growing evidence of a link between choline metabolites and cardiovascular disease (CVD) (Tang et al., 2013). However, much of the research in this area relies on study populations with a high prevalence of CVD, which may limit the generalization of the conclusions to other demographics (Shea et al., 2024).
TMAO’s role in CVD is particularly controversial. Some animal studies have shown TMAO to exacerbate atherosclerosis (Romano et al., 2015), but other studies, including observational studies in humans have failed to confirm whether TMAO is “a bystander or a mediator” in CVD (Janeiro et al., 2018; Meyer et al., 2017).
The role of choline itself is similarly blurred. This is perhaps unsurprising given that dietary sources of choline, such as red meat, are correlated with both positive and negative effects on cardiovascular health. A recent investigation using data from the Coronary Artery Risk Development in Young Adults (CARDIA) prospective cohort study found a positive association between plasma choline and CVD, independent of TMAO and betaine (Shea et al., 2024). This study also found that red meat and fried foods were significantly associated with plasma choline concentrations.
Choline’s involvement in homocysteine metabolism may offer another clue about its role in CVD, as elevated homocysteine concentrations are established risk factors for CVD (Ganguly et al., 2015). However, while choline is known to influence homocysteine regulation, there is little evidence that increasing choline consumption offers significant cardiovascular benefits through this mechanism (Meyer et al., 2017).
Choline and neurology
Choline is essential for neurological development and function (Sanders et al., 2007). Its deficiency is associated with apoptosis and neuronal cell death, potentially contributing to neurological disorders.
A recent systematic review and meta-analysis found that higher maternal choline intake was associated with positive cognitive effects and neurodevelopment in children, including memory, attention and visuospatial learning (Obeid et al., 2022).
There may also be a link between choline and cognitive function in older adults. A study of choline intake in adults aged 60 and over found that consuming at least 187.5mg per day reduced the risk of low cognitive performance by around 40% using three different cognition measures (Liu et al., 2021). Improvements tailed off at 399.5mg per day, suggesting a “U-shaped” effect with increasing choline consumption.
Cognitive impairment in older people is often caused by cerebrovascular disease, which itself may be influenced by choline levels. A 2021 study in China found that patients with higher levels of circulating choline and betaine had a reduced risk of cognitive impairment after acute ischemic stroke (Zhong et al., 2021). Earlier research suggests that CDP-choline has a neuroprotective effect in preclinical models of brain ischemia and trauma, and while findings in clinical trials have been inconclusive, there have been some promising results in cases of slower neurodegeneration, such as glaucoma and vascular cognitive impairment, and in Alzheimer’s disease (AD) (Grieb et al., 2014).
Choline supplementation and Alzheimer’s disease
Low choline intake appears to increase the risk of dementia and AD (Yuan et al., 2022). A recent metabolomic analysis found that low circulating choline levels associate with AD progression (Judd et al., 2023). A proteomics analysis of hippocampal tissue in an AD mouse model showed that choline supplementation led to changes in key proteins involved in AD pathology (Dave et al., 2023). It remains unclear whether choline supplementation can reduce neuropathology in advanced AD, but given choline’s crucial role in neurobiology, it may still be a worthwhile preventive strategy (Judd et al., 2023).
Discussing the above study from Arizona State University, lead researcher Prof. Ramon Velazquez commented, “It’s a twofold problem. First, people don’t reach the adequate daily intake of choline established by the Institute of Medicine in 1998. Second, there is vast literature showing that the recommended daily intake amounts are not optimal for brain-related functions.” The study found that choline deprivation in both healthy mice and AD-symptomatic transgenic mice altered levels of amyloid-beta protein and tau protein, which are involved in neurofibrillary tangles associated with AD (Judd et al., 2023).
Choline as a biomarker
The above associations suggest that choline may be a potential biomarker of several different diseases. Metabolomic profiling has been used to measure choline and its metabolites in pathologies such as liver dysfunction and AD (Sha et al., 2010; Donatti et al., 2020).
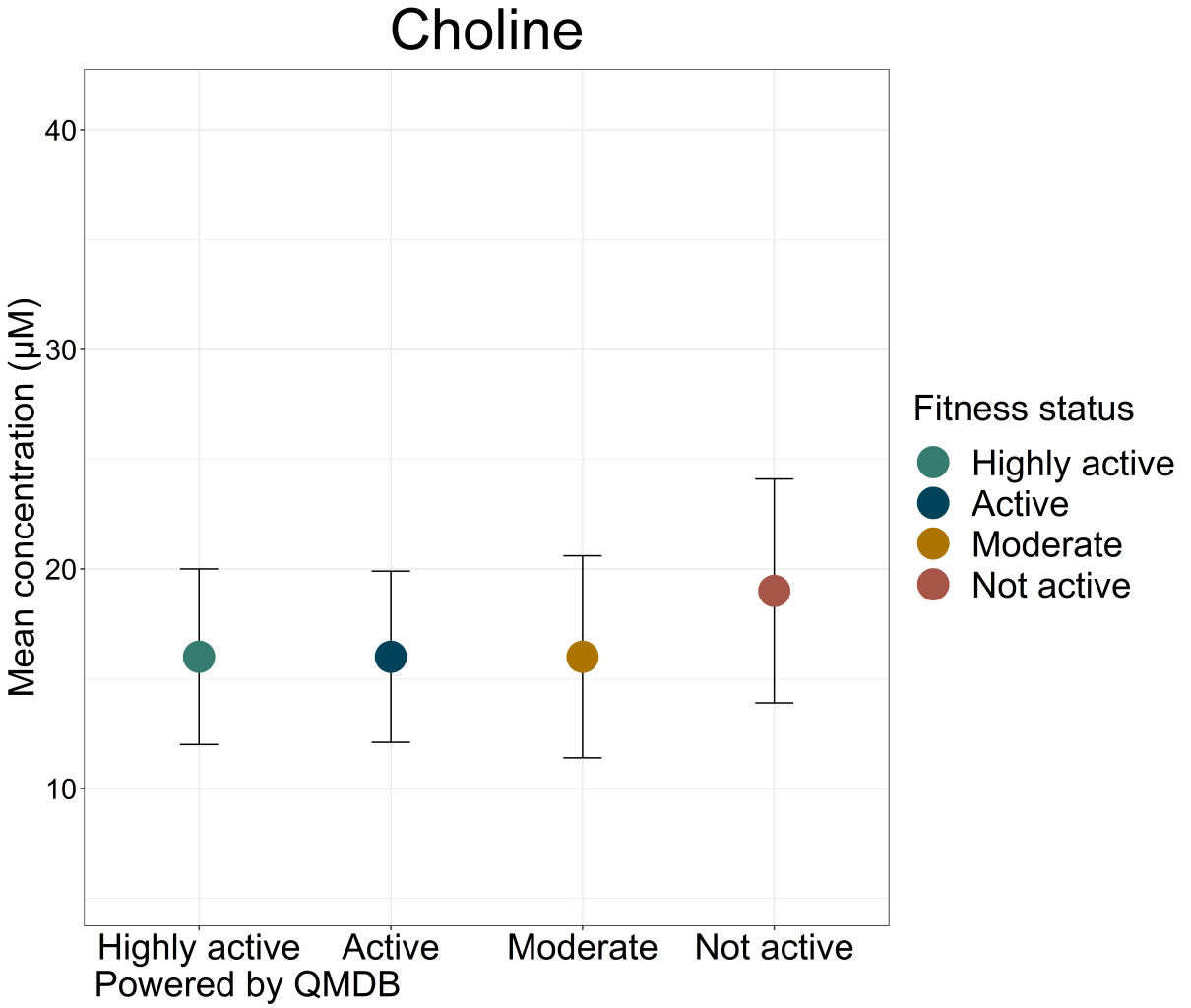
An interesting example of choline’s potential as a biomarker involves the links between smoking, choline metabolism and risk of CVD. Findings suggest that smoking alters the association between plasma choline and risk of acute myocardial infarction (AMI) (Schartum-Hansen et al., 2015).Choline levels also tend to differ in the population based on fitness or smoking status, as can be seen in biocrates’ quantitative metabolomics database (QMDB).
Potential dietary and microbiome-based interventions with choline
Despite the wide availability of choline in food, most people do not meet the daily recommended intake (Arias et al., 2020). As noted above in relation to cognitive function, supplementation may be useful in some cases.
There has been significant research in animal models. A 2023 study found that choline supplementation in pigs enhanced gut microbiome diversity and epithelial activity, which may help to promote ovarian follicular development and ovulation (Zhan et al., 2023). Another study from 2022 investigated the effects of choline supplementation on atherosclerosis in mice. It found that choline promoted beneficial changes in gut microbial composition and function, including increasing the abundance of anti-inflammatory microbiota and gene expression relating to TMA and TMAO degradation, but notably did not aggravate atherosclerosis (Liu, C. et al. 2022).
Further research in human subjects may clarify the efficacy of these and other choline-based dietary and microbiome-related interventions.
References
Arias, N. et al.: The Relationship between Choline Bioavailability from Diet, Intestinal Microbiota Composition, and Its Modulation of Human Diseases. (2020) Nutrients | DOI: doi.org/10.3390/nu12082340.
Dave, N. et al.: Dietary choline intake is necessary to prevent systems-wide organ pathology and reduce Alzheimer’s disease hallmarks. (2023) Aging Cell | DOI: doi.org/10.1111/acel.13775.
Donatti, A. et al.: Circulating Metabolites as Potential Biomarkers for Neurological Disorders—Metabolites in Neurological Disorders. (2020) Metabolites | DOI: doi.org/10.3390/metabo10100389.
Fischer, L. et al.: Dietary choline requirements of women: effects of estrogen and genetic variation. (2010) Am J Clin Nutr. | DOI: doi.org/10.3945/ajcn.2010.30064.
Ganguly, P. et al.: Role of homocysteine in the development of cardiovascular disease. (2015). Nutr J. | DOI: doi.org/10.1186/1475-2891-14-6.
Grieb, P. : Neuroprotective properties of citicoline: facts, doubts and unresolved issues. (2014) CNS Drugs | DOI: doi.org/10.1007/s40263-014-0144-8.
Griffith, W. et al.,: II. Chemistry, Ch. 5. Choline. (1954) In The Vitamins: Chemistry, Physiology, Pathology, by Sebrell Jr. W. and Harris R. New York. Academic Press Inc. sciencedirect.com/book/9781483197043/the-vitamins
Janeiro, M. et al.: Implication of Trimethylamine N-Oxide (TMAO) in Disease: Potential Biomarker or New Therapeutic Target. (2018).Nutrients | DOI: doi.org/10.3390/nu10101398.
Judd, J. et al.: Inflammation and the pathological progression of Alzheimer’s disease are associated with low circulating choline levels. (2023) Acta Neuropathol | DOI: doi.org/10.1007/s00401-023-02616-7.
Laura K. et al.: Phosphatidylcholine biosynthesis and lipoprotein metabolism, Biochimica et Biophysica Acta (BBA). (2012) Molecular and Cell Biology of Lipids | DOI: doi.org/10.1016/j.bbalip.2011.09.009
Leermakers, E. et al.: Effects of choline on health across the life course: a systematic review. (2015) Nutrition Reviews | DOI: doi.org/10.1093/nutrit/nuv010.
Li, J. et al.: Phosphatidylethanolamine N-methyltransferase: from Functions to Diseases. (2023) Aging Dis. | DOI: doi.org/10.14336/AD.2022.1025.
Liu, C. et al.: Choline and butyrate beneficially modulate the gut microbiome without affecting atherosclerosis in APOE*3-Leiden.CETP mice. (2022) Atherosclerosis | DOI: doi.org/10.1016/j.atherosclerosis.2022.10.009.
Liu, L. et al.: Choline Intake Correlates with Cognitive Performance among Elder Adults in the United States. (2021) Behav Neurol. | DOI: doi.org/10.1155/2021/2962245.
Meyer, K. et al.,: Dietary Choline and Betaine and Risk of CVD: A Systematic Review and Meta-Analysis of Prospective Studies. (2017) Nutrients | DOI: doi.org/10.3390/nu9070711.
National Institutes of Health.: Choline: Fact Sheet for Health Professionals. (2022) .Accessed March 2024. https://ods.od.nih.gov/factsheets/Choline-HealthProfessional/.
Obeid, R. et al.: Association between Maternal Choline, Fetal Brain Development, and Child Neurocognition: Systematic Review and Meta-Analysis of Human Studies. (2022).Adv Nutr. | DOI: doi.org/10.1093/advances/nmac082.
Romano, K. et al.: Intestinal Microbiota Composition Modulates Choline Bioavailability from Diet and Accumulation of the Proatherogenic Metabolite Trimethylamine-N-Oxide. (2015) ASM Journals DOI: doi.org/10.1128/mbio.02481-14
Romano, K. et al.: Intestinal Microbiota Composition Modulates Choline Bioavailability from Diet and Accumulation of the Proatherogenic Metabolite Trimethylamine-N-Oxide. (2015) ASM Journals | DOI: doi.org/10.1128/mbio.02481-14.
Sam, C. et al.,: Physiology, Acetylcholine. (2023) In: StatPearls [Internet]. FL: StatPearls Publishing. | ncbi.nlm.nih.gov/books/NBK557825/
Sanders, L. et al.,: Choline: Dietary Requirements and Role in Brain Development. (2007) Nutr Today | DOI: doi.org/10.1097/01.NT.0000286155.55343.fa.
Schartum-Hansen, H. et al.: Plasma choline, smoking, and long-term prognosis in patients with stable angina pectoris. (2015) European Journal of Preventive Cardiology 22 (5): 606–614. | DOI: doi.org/10.1177/2047487314524867.
Sha, W. et al.: Metabolomic profiling can predict which humans will develop liver dysfunction when deprived of dietary choline. (2010) FASEB J. | DOI: doi.org/10.1096/fj.09-154054.
Shea, J. et al.: Choline metabolites and incident cardiovascular disease in a prospective cohort of adults: Coronary Artery Risk Development in Young Adults (CARDIA) Study. (2024) The American Journal of Clinical Nutrition | DOI: doi.org/10.1016/j.ajcnut.2023.10.012.
Spencer, M. et al.: Association between composition of the human gastrointestinal microbiome and development of fatty liver with choline deficiency. (2010) Gastroenterology | DOI: doi.org/10.1053/j.gastro.2010.11.049.
Tang, W. et al.: Intestinal Microbial Metabolism of Phosphatidylcholine and Cardiovascular Risk. (2013) N Engl J Med | DOI: doi.org/10.1056/NEJMoa1109400.
Vance, D.: Phospholipid methylation in mammals: from biochemistry to physiological function. (2014) Biochimica et Biophysica Acta (BBA) – Biomembranes | DOI: doi.org/https://doi.org/10.1016/j.bbamem.2013.10.018.
Wallace, T. et al.: Choline: The Underconsumed and Underappreciated Essential Nutrient. (2018 ) Nutr Today. | DOI: doi.org/10.1097/NT.0000000000000302.
Yao, Z. et al., : The active synthesis of phosphatidylcholine is required for very low density lipoprotein secretion from rat hepatocytes. (1988) J Biol Chem. | pubmed.ncbi.nlm.nih.gov/3343237/
Yuan, J. et al.: Is dietary choline intake related to dementia and Alzheimer’s disease risks? Results from the Framingham Heart Study. (2022) Am J Clin Nutr. | DOI: doi.org/10.1093/ajcn/nqac193.
Zeisel, S.: A brief history of choline. (2012) Ann Nutr Metab. | DOI: doi.org/10.1159/000343120.
Zhan, X. et al.: Choline supplementation regulates gut microbiome diversity, gut epithelial activity, and the cytokine gene expression in gilts. (2023) Front. Nutr. | DOI: doi.org/10.3389/fnut.2023.1101519.
Zhong, C. et al.: Choline Pathway Nutrients and Metabolites and Cognitive Impairment After Acute Ischemic Stroke. (2021) | DOI: doi.org/10.1161/STROKEAHA.120.031903.