History & Evolution
1814-23: first observed in impure form by Michel Eugène Chevreul (Chevreul 1823) | 1869: first synthesized by Lieben and Rossi (Goldberg 2009)
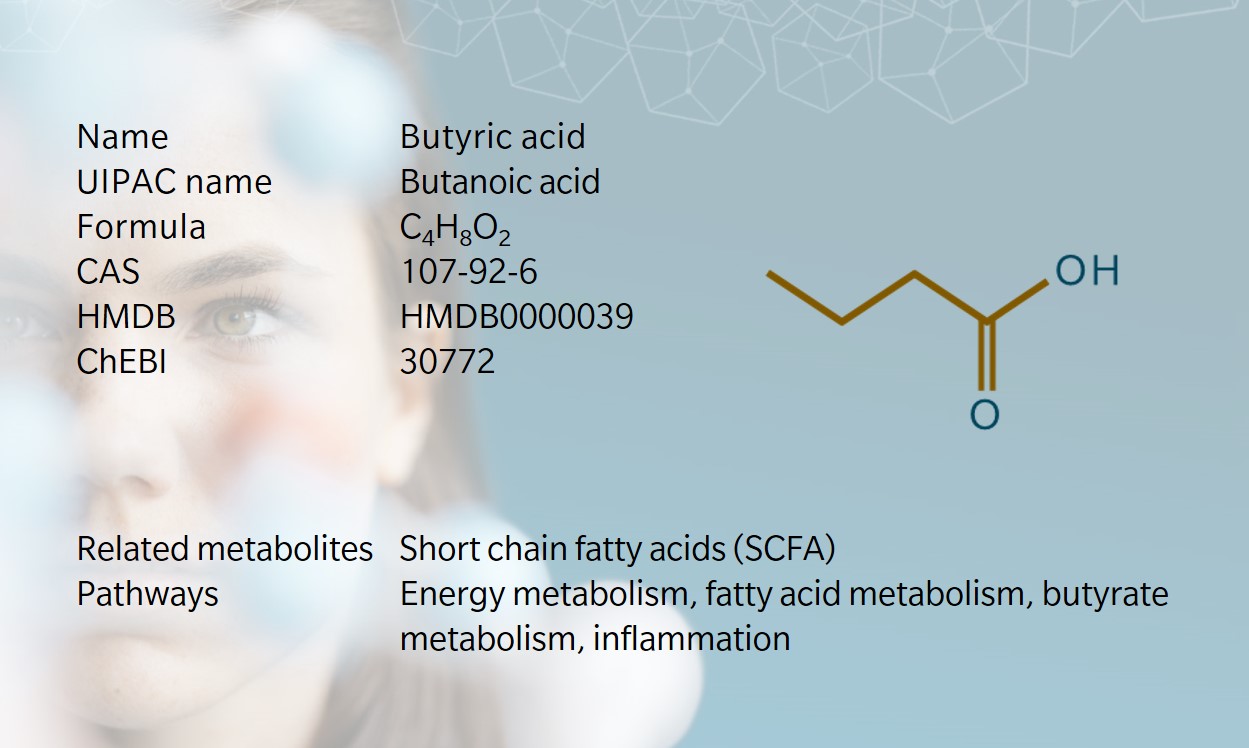
Butyric acid is a four-carbon straight short chain fatty acid (SCFA) found in the esters of animal fats and plant oils. Its name comes from the Ancient Greek for butter, which is where it was first identified. Butyric acid is responsible for the foul smell found in rancid butter, parmesan cheese, vomit and body odor (Huang et al. 2018). Interestingly, as for isovaleric acid, some esters of butyric acid have a more appealing scent, and are often used in perfumes.
Butyric acid is one of three common SCFAs in the human gut, alongside acetic acid and propionic acid, which together make up 90-95% of the SCFAs in the colon (Ríos-Cavián et al. 2016). It is a major source of energy for the colon and is used in treatments for colorectal cancer, hemoglobinopathies and gastrointestinal diseases (Huang et al. 2018).
In industry, butyric acid has applications in food, textile production, animal feed, and biofuels, often chemically synthesized through the oxidation of propylene-derived butyraldehyde, or through syngas fermentation (Huang et al. 2018).
Biosynthesis vs. dietary uptake

Most SCFAs in the gut come from dietary fibers: because humans lack the enzymes to digest these, they pass through the intestinal tract and are fermented by host bacteria. Butyric acid is a conjugate of butyrate, which is produced through the fermentation of hydrolysis-resistant starches and dietary fiber by anaerobic bacteria in the colon (Wong et al. 2006). Some butyrate is also produced as proteins and peptides are digested in the bowel (Macfarlane et al. 2003).
Diet, composition of the microbiome, and intestinal transit time all influence butyric acid formation, as with the other SCFAs (Morrison et al. 2016). Most of the dietary fiber from which butyric acid is produced comes from plant sources, such as resistant starch, cruciferous vegetables, and foods with a high sulphur content (Rivière et al. 2016). Dietary butyric acid is found in dairy products, red meat, and fermented foods such as sauerkraut. Around 5% of the saturated fat in dairy products comes from butyric acid (Månsson 2008). Butyric acid can also be taken in supplement form.
Butyric acid and the microbiome
SCFAs are a popular research topic in medical biochemistry because of their potential role in gut function, glucose homeostasis, metabolic regulation, and appetite (Blakeney et al. 2019; Vijay et al. 2021). They are also known to influence inflammation and immune response.
In the colon, butyrate is a source of energy for endothelial cells, promotes cell differentiation and apoptosis, and can inhibit colonic acidification (Wong et al. 2006). Some studies suggest that butyrate can suppress colorectal cancer, though results are inconclusive (Silva et al. 2020). Butyric acid has been shown to influence pathogenesis of gastrointestinal disease and gut dysbiosis, and animal studies show that higher concentrations of butyric acid in the colon reduce the severity of inflammation.
Butyric acid as a signaling molecule
SCFAs are known to act as signaling molecules between gut microbiota and host, with receptors in many different cell and tissue types (Morrison et al. 2016). Butyric acid is an endogenous agonist of one of these receptors, hydroxycarboxylic acid receptor 2 (HCA2). HCA2 is a protein receptor that can inhibit the breakdown of fats, giving butyric acid a key role in lipid metabolism. Butyric acid is also an agonist of the peroxisome proliferator-activated receptor (PPAR), a nutrient sensor which helps to stabilize lipid metabolism and inhibit cancer cell proliferation in the colon (Hong et al. 2019).
One notable way in which butyric acid regulates the inflammatory process is by stimulating the production of eicosanoids, which are lipid mediators derived from arachidonic acid (Vinolo et al. 2011). These are also known to regulate other immune processes involved in cancer, asthmas, and arthritis (Harizi et al. 2008).
Butyric acid and the immune system
As noted, butyric acid exerts several effects in the human gut which affect immune processes (Kovarik et al. 2013). Butyric acid is thought to increase acetylation of histone H3, in turn influencing the behavior of regulatory T cells, which can inhibit the immune response (Borycka-Kiciak et al. 2017). Through this mechanism, SCFAs link crosstalk between the human microbiome and immune system, though it is not clear whether this is by increasing tolerance in the microbiome, or by reducing the inflammatory response (Morrison et al. 2016).
Recent research has highlighted the significance of butyric acid in the gut microbiome, particularly its role in maintaining immune function and metabolic balance. For instance, a study investigating age-associated gut dysbiosis in older individuals living with HIV found a notable decrease in butyrogenic potential, correlating with alterations in plasma tryptophan metabolites (Brivio et al. 2023)
A few clinical studies have observed an anti-inflammatory effect from the therapeutic use of SCFAs in cases of inflammatory bowel disease, radiation proctitis, and diabetes. Growing evidence suggests SCFAs support the immune system and metabolism through gut-liver inflammatory pathways (Morrison et al. 2016).
Butyric acid and the brain
In addition to its role in the gastrointestinal tract, butyric acid may also contribute to links between gut dysbiosis and neurological conditions, such as depression, Alzheimer’s disease, Parkinson’s disease, and autism spectrum disorder (Silva et al. 2020).
Studies looking at the use of probiotics to increase butyrate-producing bacteria in the gut suggest butyrates could help reduce anxiety and lower stress (Bourassa et al. 2016). A review by Bourassa et al. proposed possible mechanisms for butyric acid’s neuroprotective effects, including mitochondrial activity, G-protein coupled receptors, histone acetylation, and microbiome homeostasis. A clear line was drawn between the consumption of a high fiber diet, butyrate production, and protection against multiple neurological conditions through these pathways (Bourassa et al. 2016).
Butyric acid and cardiometabolic diseases

The role of SCFAs in lipid and energy metabolism links them to certain metabolic conditions. Butyrate has been shown to protect against diet-induced obesity and insulin resistance, which suggests it may offer potential therapeutic role in obesity-related diseases and diabetes (Lin et al. 2012). Animal studies confirm that butyric acid supplementation can improve insulin sensitivity: in one study, butyric acid caused fat loss and improved insulin tolerance in mice (Heimann et al. 2015, Gao et al. 2009). More research is needed to confirm the effect in humans.
Gut microbiota have a well-established link to coronary artery disease and atherosclerosis. One animal study has shown that butyrate supplementation could reduce atherosclerotic lesions, while another suggested that butyric acid seems to mediate gut microbiota and the circulatory system (Onyszkiewicz et al. 2019). Some studies have suggested that butyric acid affects arterial blood pressure, with one showing a significant hypotensive effect when butyric acid concentration in the colon was increased (Onyszkiewicz et al. 2019). The precise mechanism is unknown: it may result from bacterial metabolites having a stimulating enterosyne effect on the enteric nervous system, or from metabolite-derived molecules entering the circulatory system and influencing arterial blood pressure through various organs (Onyszkiewicz et al. 2019).
Butyric acid and cancer: the “butyrate paradox”
As noted, butyric acid has been shown in several studies to inhibit the proliferation of cancer cells in the colon, by inducing apoptosis, inhibiting cancer gene expression, inhibiting cancer cell proliferation, and promoting anti-inflammatory processes (Williams et al. 2007). However, other studies challenge the notion of a chemopreventive effect from butyrate, and there is a lack of agreement particularly when comparing in vitro and in vivo studies, referred to as the butyrate paradox (Lupton 2004). It seems likely that butyrate’s chemopreventive effect depends on the amount of butyrate, time of exposure during the tumorigenic process, and type of dietary fat. Our understanding of the underlying molecular mechanisms is likely to grow with the advance of genomic and metabolomic technologies. Because butyric acid is a by-product of fiber fermentation, this could explain why high fiber diets help to protect against colorectal cancer, as well as obesity, stroke, type 2 diabetes and other conditions.
Learn more about the roles of butyric acid and other SCFAs in complex chronic diseases such as cancer, Alzheimer’s disease, depression, inflammatory bowel disease, multiple sclerosis and diabetes in our whitepaper “Complex chronic diseases have a common origin”.
References
Blakeney, B. et al.: The Branched Short Chain Fatty Acid Isovaleric Acid Causes Colonic Smooth Muscle Relaxation via cAMP/PKA Pathway. (2019) Digestive Diseases and Sciences | https://doi.org/10.1007/s10620-018-5417-5
Borycka-Kiciak, K. et al.: Butyric acid – a well-known molecule revisited. (2017) Gastroenterology Review | https://doi.org/10.5114/pg.2017.68342
Bourassa, M. et al.: Butyrate, Neuroepigenetics and the Gut Microbiome: Can a High Fiber Diet Improve Brain Health? (2016) Neuroscience Letters | https://doi.org/10.1016/j.neulet.2016.02.009
Brivio, P et al.: Venlafaxine’s effect on resilience to stress is associated with a shift in the balance between glucose and fatty acid utilization (2023) Neuropsychopharmacol. 48, 1475–1483). | https://doi.org/10.1038/s41386-023-01633-0
Chevreul, M.: Recherches Chimiques sur les Corps Gras d’Origine Animale. (1823) Paris: Imprimerie Nationale | https://gallica.bnf.fr/ark:/12148/bpt6k9671903v/f459.item
Gao, Z. et al.: Butyrate Improves Insulin Sensitivity and Increases Energy Expenditure in Mice. (2009) Diabetes | https://doi.org/10.2337/db08-1637
Goldberg, I. and Rokem, JS.: Organic and Fatty Acid Production (2009) Microbial. Vol. 3rd edition, in Encyclopedia of Microbiology, by Moselio Schaechter | https://doi.org/10.1016/B978-0-12-809633-8.13083-3
Harizi, H. et al.: Arachidonic-acid-derived eicosanoids: roles in biology and immunopathology. (2008) Trends in Molecular Medicine | https://doi.org/10.1016/j.molmed.2008.08.005
Heimann, E. et al.: Propionic acid and butyric acid inhibit lipolysis and de novo lipogenesis and increase insulin-stimulated glucose uptake in primary rat adipocytes. (2015) Adipocyte | https://doi.org/10.4161/21623945.2014.960694
Hong, F. et al.: PPARs as Nuclear Receptors for Nutrient and Energy Metabolism. (2019) Molecules | https://doi.org/10.3390/molecules24142545
Huang, J. et al.: Biosynthesis of butyric acid by Clostridium tyrobutyricum. (2018) Preparative Biochemistry & Biotechnology | https://doi.org/10.1080/10826068.2018.1452257
Kovarik, K. et al.: Eicosanoid modulation by the short-chain fatty acid n-butyrate in human monocytes. (2013) Immunology | https://doi.org/10.1111/imm.12089
Lin, H. et al.: Butyrate and Propionate Protect against Diet-Induced Obesity and Regulate Gut Hormones via Free Fatty Acid Receptor 3-Independent Mechanisms. (2012) PLoS One | https://doi.org/10.1371/journal.pone.0035240
Lupton, J.: Microbial Degradation Products Influence Colon Cancer Risk: the Butyrate Controversy. (2004) The Journal of Nutrition | https://doi.org/10.1093/jn/134.2.479
Macfarlane, G. and Macfarlane, T.: Regulation of short-chain fatty acid production. (2003) Proceedings of the Nutrition Society | https://doi.org/10.1079/PNS2002207
Månsson, HL.: Fatty acids in bovine milk fat. (2008) Food and Nutrition Research | https://doi.org/10.3402/fnr.v52i0.1821
Morrison, D. and Preston T.: Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. (2016) Gut Microbes | https://doi.org/10.1080/19490976.2015.1134082
Onyszkiewicz, M. et al.: Butyric acid, a gut bacteria metabolite, lowers arterial blood pressure via colon-vagus nerve signaling and GPR41/43 receptors. (2019) Pflügers Archiv – European Journal of Physiology | https://doi.org/10.1007/s00424-019-02322-y
Ríos-Cavián, D. et al.: Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health.(2016) Frontiers in Microbiology | https://doi.org/10.3389/fmicb.2016.00185
Rivière, A. et al.: Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. (2016) Frontiers in Microbiology | https://doi.org/10.3389/fmicb.2016.00979
Silva, Y. et al.: “The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. (2020) Frontiers in Endocrinology | https://doi.org/10.3389/fendo.2020.00025
Vijay A. Et al.: The prebiotic effects of omega-3 fatty acid supplementation: A six-week randomised intervention trial (2021) Gut Microbes 13(1):1-11 | DOI: 10.1080/19490976.2020.1863133
Vinolo, M. et al.: Regulation of Inflammation by Short Chain Fatty Acids.” Nutrients (2011) | https://doi.org/10.3390/nu3100858
Williams, E. et al.: Anti-cancer effects of butyrate: use of micro-array technology to investigate mechanisms. (2007) Proceedings of the Nutrition Society | https://doi.org/10.1079/PNS2002230
Wong, J. et al.: Colonic health: fermentation and short chain fatty acids. (2006) Journal of Clinical Gastroenterology | https://doi.org/10.1097/00004836-200603000-00015