- History & Evolution
- Biosynthesis & dietary uptake
- Methylmalonic acid and the microbiome
- Methylmalonic acid and vitamin B12 deficiency
- Methylmalonic acid and neurology
- Methylmalonic acid and 5P medicine
- References
History & Evolution
1963: First clinical description in pernicious anemia | 1967: Discovery of methylmalonic aciduria | 1968: Vitamin B12 responders in methylmalonyl-CoA deficiency | 1993: Methylmalonic acid (MMA) as biomarker for vitamin B12 deficiency
Methylmalonic acid (MMA) was first linked to human disease in 1963, when researchers observed elevated urinary levels in patients with pernicious anemia (Barness et al. 1963), a rare and fatal disease marked by progressive anemia and neurological decline (American Chemical Society 2025). In 1967, MMA was identified as the hallmark of a newly described inborn error of metabolism, methylmalonic aciduria (Oberholzer et al. 1967). The underlying cause—methylmalonyl-CoA mutase deficiency—was discovered soon after in 1968 (Rosenberg et al. 1968a). However, not all cases were due to a defect in the enzyme itself. Some patients showed a biochemical response to vitamin B12 supplementation, leading to the identification of B12-responsive subtypes (Rosenberg et al. 1968b).
Accordingly, in 1993, MMA was established as a sensitive biomarker for detecting early or subclinical vitamin B12 deficiency, often before B12 levels dropped (Allen et al. 1993). Recognizing its diagnostic value, MMA measurement was added to the U.S. newborn screening panel in 2006 to detect methylmalonic acidemia. More recently, large population studies linked elevated MMA to cognitive decline (Clarke et al. 2007), depressive symptoms and increased mortality (Cao et al. 2024), underscoring its broader relevance in aging and public health (Tejero et al. 2024).
Biosynthesis vs. dietary uptake
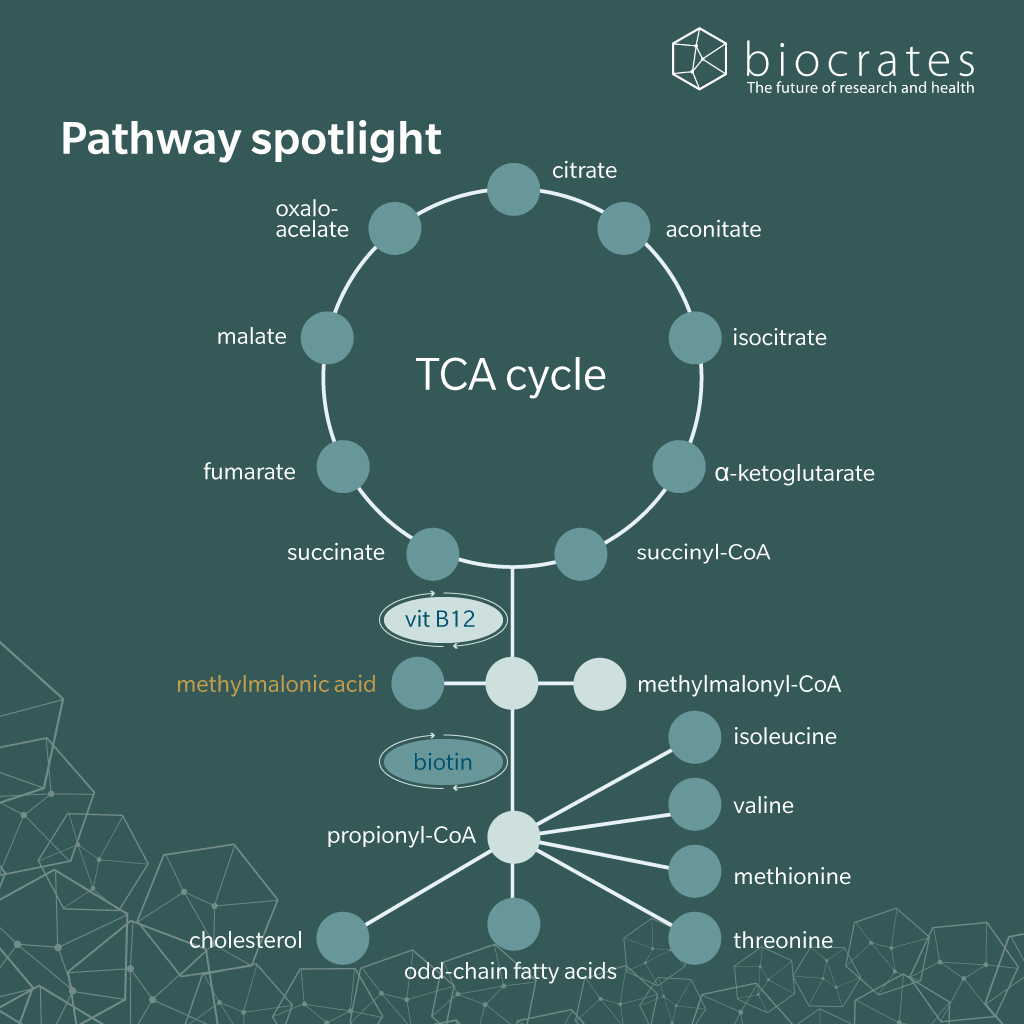
MMA is a short-chain dicarboxylic acid and an intermediate in the catabolism of certain amino acids, fatty acids and cholesterol. It is not obtained from the diet but is synthesized endogenously as part of normal human metabolism. The primary biosynthetic route to MMA occurs in the mitochondria through the conversion of propionyl-coenzyme A (CoA).
In this pathway, propionyl-CoA is first carboxylated to D-methylmalonyl-CoA by propionyl-CoA carboxylase, a biotin-dependent enzyme. D-methylmalonyl-CoA is then converted to its L-isomer by methylmalonyl-CoA epimerase (Tejero et al. 2024). Subsequently, the L-isomer is converted to succinyl-CoA by methylmalonyl-CoA mutase (MUT), a mitochondrial enzyme that requires the active coenzyme form of vitamin B12, adenosylcobalamin (Takahashi-Iñiguez et al. 2012). Finally, the resulting succinyl-CoA enters the tricarboxylic acid cycle (Tejero et al. 2024).
When MUT activity is impaired due to genetic mutations or functional vitamin B12 deficiency, methylmalonyl-CoA cannot be efficiently converted into succinyl-CoA. As a result, it accumulates and is hydrolyzed to free MMA, which then appears in plasma (Allen et al. 1993).
Under normal physiological conditions, plasma MMA concentrations are low (typically <0.3 µmol/L in adults), reflecting efficient MUT activity and adequate cobalamin status. Elevated MMA is one of the earliest and most specific indicators of intracellular B12 deficiency, and unlike homocysteine, its levels are not influenced by folate or vitamin B6 status (O’Leary et al. 2010).
Once produced, MMA is not reused; instead, it is filtered by the kidneys and eliminated in urine. Renal function therefore plays a critical role in regulating plasma MMA levels, and mild elevations can also be observed in renal impairment (Supakul et al. 2020).
Methylmalonic acid and the microbiome
Emerging evidence suggests that circulating levels of MMA can be shaped by the gut microbiome, complicating their use as a straightforward biomarker of B12 deficiency in clinical settings.
Recent studies have shown that certain gut microbial communities are significantly associated with serum MMA concentrations, independently of host B12 status. For example, in healthy adults, oral antibiotic treatment led to a reduction in serum MMA, implying that gut bacteria contribute to its circulating pool (Miller et al. 2025).
Microbial co-abundance guilds (groups of taxa with correlated abundance patterns) have been found to associate with either serum MMA or B12, but not both. This suggests that different microbial profiles influence MMA through mechanisms beyond B12 availability, such as enhanced propionate production or altered short-chain fatty acid (SCFA) metabolism (Miller et al. 2025). Among SCFAs, propionate uniquely accounted for a significant portion of the variance in MMA levels, further highlighting the specificity of this microbial-metabolite link.
The clinical relevance of this microbiota–MMA axis has become particularly evident in metabolic disease. In gestational diabetes mellitus (GDM), integrated multiomics approaches have revealed close correlations between the fecal microbiota and plasma metabolome. Specifically, plasma levels of MMA were strongly associated with alterations in gut microbiota composition (Dong et al. 2020).
Moreover, case reports in patients with small intestinal bacterial overgrowth further support a microbial contribution to elevated MMA: in such individuals, broad-spectrum antibiotics normalized MMA levels, suggesting that gut bacteria capable of producing propionate—and potentially MMA itself—can significantly elevate systemic concentrations (Tejero et al. 2024).
Together, these findings challenge the long-held assumption that elevated MMA is a direct marker of B12 deficiency. Instead, gut microbial activity, particularly through propionate-producing pathways, may represent an additional, independent source of MMA in the circulation of the host.
Methylmalonic acid and vitamin B12 deficiency
Despite growing interest in its microbial origins, MMA’s primary clinical role remains as the most specific functional marker of vitamin B12 (cobalamin) status. When B12 is insufficient at the cellular level, related metabolic processes begin to fail, such as the conversion of methylmalonyl-CoA to succinyl-CoA. As a result, MMA levels in the blood often increase before B12 falls below the classical deficiency cut-off of 200 pg/mL, making it a useful marker of “functional” deficiency in patients whose total B12 level remains within the low-normal range (201–350 pg/mL) (Ankar et al. 2025; Office of Dietary Supplements 2024).
In a 2015 article, Fedosov et al. recommend the inclusion of MMA as part of a comprehensive diagnostic panel alongside total serum B12, holotranscobalamin (active B12) and homocysteine to monitor B12 status (Fedosov et al. 2015).
Functionally, MMA is more than a diagnostic tool: it is implicated in the pathology of B12 deficiency. Elevated MMA disrupts normal lipid metabolism and mitochondrial energy production, contributing to myelin sheath instability and neurotoxicity (Ankar et al. 2025). These mechanisms are thought to underlie many of the neurological symptoms associated with B12 deficiency, including peripheral neuropathy, ataxia, cognitive dysfunction and dementia-like presentations (Langan et al. 2017; Brito et al. 2017; Ankar et al. 2025)
Methylmalonic acid and neurology
MMA accumulation following decreased conversion of methylmalonyl-CoA to succinyl-CoA also impairs TCA cycle flux. This contributes to mitochondrial dysfunction and promotes oxidative stress through inhibition of respiratory chain complexes and lipid peroxidation. These effects are particularly damaging to the central nervous system, which relies heavily on mitochondrial ATP production and intact myelin structure for normal function (Denley et al. 2025).
High MMA levels associate with a range of neurological conditions, including depression and cognitive impairment, as well as increased risk of neurodegenerative disease. In a large cross-sectional analysis of the U.S. National Health and Nutrition Examination Survey (NHANES) data, higher circulating MMA was independently associated with increased depressive symptoms and a ~25% increase in all-cause mortality, even after adjusting for serum B12 and folate levels (Cao et al. 2024). Additional analyses have confirmed that elevated MMA is more prevalent among individuals with dementia and Alzheimer’s disease than in cognitively healthy controls (Refsum et al. 2003; O’Leary et al. 2012). Clinical observations also suggest a high co-occurrence of elevated MMA and subclinical B12 deficiency in patients presenting with neuropsychiatric complaints, including memory loss, fatigue and mood disorders (Nalder et al. 2021; Moore et al. 2012)
Longitudinal data support these findings. In the Oxford Age and Nutrition Project, doubling of baseline MMA concentrations (from 0.25 to 0.50 µM) was associated with a 50% faster rate of cognitive decline (Clarke et al. 2007). Similarly, the Chicago Memory and Aging Project found that higher MMA levels predicted steeper six-year decline in memory and executive function (Tangney et al. 2009). In children, elevated MMA was also linked to reduced cognitive performance in later years (Kvestad et al. 2017).
In a clinical trial involving elderly Chilean participants with vitamin B12 deficiency and elevated levels of MMA, B12 repletion led to increased serum concentrations of acylcarnitines, plasmalogens, phospholipids, and decreased methionine and cysteine. This metabolite shifts are indicative of enhanced mitochondrial function and one-carbon metabolism and coincided with improved peripheral nerve conduction, suggesting a direct link between B12 status, metabolic repair and neural function (Brito et al. 2017).
Methylmalonic acid and aging
MMA accumulates progressively with age, even in individuals without overt B12 deficiency or renal dysfunction (O’Leary et al., 2012; Clarke et al., 2007). This age-related rise in MMA has been linked to cognitive decline, physical frailty and increased mortality (Cao et al., 2024; Tangney et al., 2009).
Mechanistically, elevated MMA interferes with mitochondrial function, impairs cellular energy metabolism, promotes oxidative stress and triggers pro-inflammatory signaling (Tejero et al. 2024). These effects mirror several hallmarks of aging, including mitochondrial dysfunction, chronic inflammation and disrupted intercellular communication (López-Otín et al. 2023).
Further findings also connect MMA to cancer progression. In elderly individuals, elevated circulating MMA creates a systemic environment that promotes tumor aggressiveness by inducing transcriptional programs such as SRY-Box Transcription Factor 4 (SOX4) and driving processes like epithelial-to-mesenchymal transition (Gomes et al. 2020). This positions MMA as a molecular bridge between aging and cancer biology, suggesting that targeting MMA accumulation may offer a novel strategy to counteract both age-related decline and tumor progression.
Methylmalonic acid and 5P medicine
MMA is a valuable tool in predictive and preventive medicine. As one of the earliest markers of functional vitamin B12 deficiency, rising MMA levels can signal risk for neurological decline, frailty and even cancer progression, often before symptoms appear. When combined with genetic or metabolic information, MMA helps deliver precision medicine, distinguishing between inherited disorders, functional B12 deficiency or microbiome-related causes. This allows for early, targeted interventions such as B12 supplementation or microbiome-based strategies to prevent further damage. Finally, MMA empowers both clinicians and patients to take informed action, shifting care from reaction to prevention.
References
Allen, R.H. et al.: Metabolic abnormalities in cobalamin (vitamin B12) and folate deficiency (1993) FASEB journal : official publication of the Federation of American Societies for Experimental Biology | DOI: 10.1096/fasebj.7.14.7901104.
Ankar, A. et al.: Vitamin B12 Deficiency (2025) | https://www.ncbi.nlm.nih.gov/books/NBK441923/.
Barness, L.A. et al.: Methylmalonate excretion in a patient with pernicious anemia (1963) The New England journal of medicine | DOI: 10.1056/NEJM196301172680309.
Brito, A. et al.: The Human Serum Metabolome of Vitamin B-12 Deficiency and Repletion, and Associations with Neurological Function in Elderly Adults (2017) The Journal of nutrition | DOI: 10.3945/jn.117.248278.
Cao, B. et al.: Associations of methylmalonic acid and depressive symptoms with mortality: a population-based study (2024) Translational Psychiatry | DOI: 10.1038/s41398-024-03015-6.
Clarke, R. et al.: Low vitamin B-12 status and risk of cognitive decline in older adults (2007) The American Journal of Clinical Nutrition | DOI: 10.1093/ajcn/86.5.1384.
Denley, M.C.S. et al.: Mitochondrial dysfunction drives a neuronal exhaustion phenotype in methylmalonic aciduria (2025) Communications Biology | DOI: 10.1038/s42003-025-07828-z.
Dong, L. et al.: Integrated microbiome-metabolome analysis reveals novel associations between fecal microbiota and hyperglycemia-related changes of plasma metabolome in gestational diabetes mellitus (2020) RSC advances | DOI: 10.1039/C9RA07799E.
Fedosov, S.N. et al.: Combined indicator of vitamin B12 status: modification for missing biomarkers and folate status and recommendations for revised cut-points (2015) Clinical chemistry and laboratory medicine | DOI: 10.1515/cclm-2014-0818.
Gomes, A.P. et al.: Age-induced accumulation of methylmalonic acid promotes tumour progression (2020) Nature | DOI: 10.1038/s41586-020-2630-0.
Kvestad, I. et al.: Vitamin B-12 status in infancy is positively associated with development and cognitive functioning 5 y later in Nepalese children (2017) The American Journal of Clinical Nutrition | DOI: 10.3945/ajcn.116.144931.
Langan, R.C. et al.: Vitamin B12 Deficiency: Recognition and Management (2017) American family physician | PMID: 28925645.
López-Otín, C. et al.: Hallmarks of aging: An expanding universe (2023) Cell | DOI: 10.1016/j.cell.2022.11.001.
Miller, J.W. et al.: Serum Methylmalonic Acid Is Associated With Gut Microbial Co-Abundance Guilds and Circulating Propionate in Healthy Adults (2025) Current Developments in Nutrition | DOI: 10.1016/j.cdnut.2025.107421.
Moore, E. et al.: Cognitive impairment and vitamin B12: a review (2012) International psychogeriatrics | DOI: 10.1017/S1041610211002511.
Nalder, L. et al.: Vitamin B12 and Folate Status in Cognitively Healthy Older Adults and Associations with Cognitive Performance (2021) The journal of nutrition, health & aging | DOI: 10.1007/s12603-020-1489-y.
Oberholzer, V.G. et al.: Methylmalonic aciduria. An inborn error of metabolism leading to chronic metabolic acidosis (1967) Archives of disease in childhood | DOI: 10.1136/adc.42.225.492.
Office of Dietary Supplements: Office of Dietary Supplements – Vitamin B12 (2024) | https://ods.od.nih.gov/factsheets/VitaminB12-HealthProfessional/.
O’Leary, F. et al.: Vitamin B12 in health and disease (2010) Nutrients | DOI: 10.3390/nu2030299.
O’Leary, F. et al.: Vitamin B₁₂ status, cognitive decline and dementia: a systematic review of prospective cohort studies (2012) British Journal of Nutrition | DOI: 10.1017/S0007114512004175.
Refsum, H. et al.: Low vitamin B-12 status in confirmed Alzheimer’s disease as revealed by serum holotranscobalamin (2003) Journal of neurology, neurosurgery, and psychiatry | DOI: 10.1136/jnnp.74.7.959.
Rosenberg, L.E. et al.: Methylmalonic aciduria. An inborn error leading to metabolic acidosis, long-chain ketonuria and intermittent hyperglycinemia (1968a) The New England journal of medicine | DOI: 10.1056/NEJM196806132782404.
Rosenberg, L.E. et al.: Methylmalonic aciduria: metabolic block localization and vitamin B 12 dependency (1968b) Science (New York, N.Y.) | DOI: 10.1126/science.162.3855.805.
Supakul, S. et al.: Diagnostic Performances of Urinary Methylmalonic Acid/Creatinine Ratio in Vitamin B12 Deficiency (2020) Journal of Clinical Medicine | DOI: 10.3390/jcm9082335.
Takahashi-Iñiguez, T. et al.: Role of vitamin B12 on methylmalonyl-CoA mutase activity (2012) Journal of Zhejiang University. Science. B | DOI: 10.1631/jzus.B1100329.
Tangney, C.C. et al.: Biochemical indicators of vitamin B12 and folate insufficiency and cognitive decline (2009) Neurology | DOI: 10.1212/01.wnl.0000341272.48617.b0.
Tejero, J. et al.: Methylmalonic acid in aging and disease (2024) Trends in endocrinology and metabolism: TEM | DOI: 10.1016/j.tem.2023.11.001.