This is the third in a series of five blogs where I’ll discuss how metabolomics is set to transform medicine as we know it. The applications of omics in biomedical research are vast, and to help organize the different ways metabolomics can be used, I’ll discuss this in the context of 5P medicine.
If you are already familiar with the concept of 5P medicine and how omics contribute to the transformation of medicine, click to move forward to the precision medicine section of this blog.
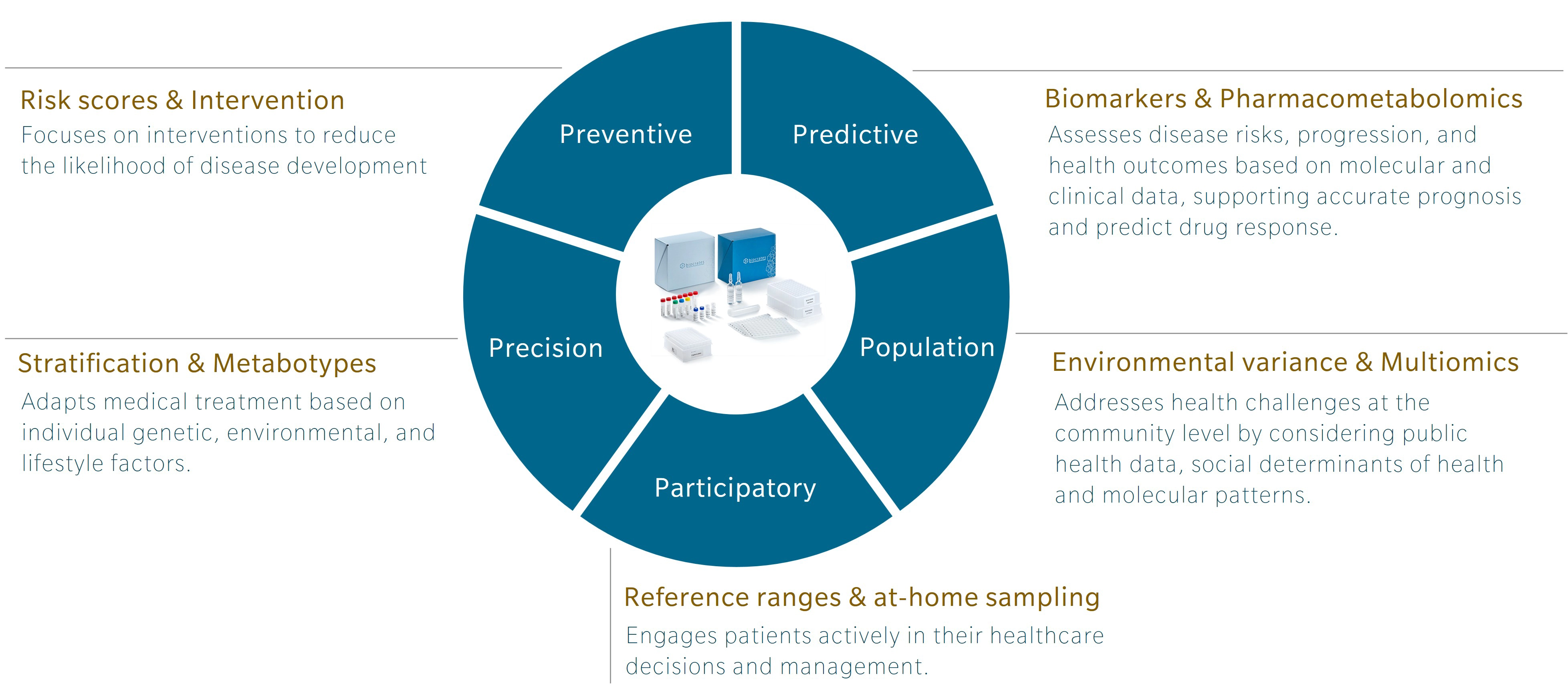
An introduction to 5P medicine
5P medicine is a concept developed to address the limitations of traditional Western medicine, which typically focuses on reacting to illness or injury. Making use of five components – preventive, predictive, precision, participatory and population-based medicine – 5P medicine aims to shift the focus towards a more proactive and patient-centric practice.
Personally, I first encountered this concept in a book by Leroy Hood and Nathan Price, The Age of Scientific Wellness. Hood and Price describe what they call P4 medicine. Rather than the familiar model that treats or manages disease after its occurrence, the authors offer an alternative that leverages the scientific tools at our disposal to understand health and disease. The result is a transition from what they call a “sickcare” system towards a genuine “healthcare” system. The 5P model expands this concept by adding population-based medicine to the original four and incorporating strategies that use the power of large cohort studies to find additional insights.
Omics and the future of research and health
Omics research has been around for over 30 years, and while genomics is gaining traction and beginning to be used in the clinics, other omics, and multiomics integration, are still lagging. Each omic addresses a distinct layer of biology, with its own codes, regulatory signals and sensitivity to external influences that offer unique insights.
Genomics is the layer least influenced by environment once a person is born. Of course, mutations can occur and change someone’s DNA, but these happen locally and are most often corrected. In contrast, metabolomics is the layer most sensitive to the environment. It responds to our diet, lifestyle, and exposures. For example, metabolomics profiles can shift in response to year after year of terrible food choices (Limonciel et al. 2013). Poor diet usually leads to chronic low-grade inflammation, which presents metabolic patterns closely associated with markers of inflammation at the granular level (Pietzner et al. 2017).
Genomics can tell you about your risk of developing an inflammatory disease, but it cannot track how this risk evolves throughout your life. Similarly, genomics can identify genotypes that will influence response to a specific drug, but it does not respond to influences from the environment that determine our drug response. Metabolomics can. That’s exactly why it’s such a great tool for precision medicine, from the identification of personalized therapeutics and nutrition to the stratification of groups of patients into phenotypically-relevant sub-groups (Wishart 2016).
Learn more about the power of multiomics for 5P medicine in my webinar.
Precision medicine with metabolomics
Precision medicine is a medical approach that tailors disease prevention, diagnosis and treatment to the individual characteristics of a patient or sub-group of patients. By focusing on factors such as their genetics, environment, lifestyle, and molecular profile, this approach aims to achieve the most effective and targeted healthcare outcomes.
I consider data disaggregation (or stratification) by sex or gender as ground zero of precision medicine. There are such marked differences between the metabolomes of women and men that it is essential to study them as two separate groups. In fact, I dedicated an entire chapter of my book on metabolomics data interpretation, The STORY principle, to this topic. Studying women and men independently helps ensure that meaningful differences in the data aren’t averaged out or overlooked when looking for diagnostic tools. It also enables the development more precisely tailored treatment regimens, or even entirely different drugs, that address disease through the specific biological mechanisms active in each group.
This level of detail becomes possible with metabolomics. A person’s metabolome provides a wealth of information that enables personalized diagnostics, treatment optimization, nutritional guidance.
Personalized prognostic and diagnostics
To refine our prognostic and diagnostic tools and to provide tailored support to patients, we need more precise measures of disease risk and disease state.
The 2020 paper by Arnold et al. “Sex and APOE ε4 genotype modify the Alzheimer’s disease serum metabolome” illustrates the differences between women and men in a disease that primarily affects women. Even when studying individuals with the strongest genetic risk factor for the disease, the differences between the sexes were striking, with women showing a stronger impact on mitochondrial energy production. I discuss this paper, and the added value of stratifying data by sex, in more detail in my conversation with Gabi Kastenmüller on The Metabolomist.
In 2021, a multiomics analysis of the blood of patients with Alzheimer’s disease uncovered molecular subtypes and regulatory mechanisms of the disease (Horgusluoglu et al. 2021). Combining genomics, transcriptomics, proteomics and metabolomics, the authors created a comprehensive molecular map of Alzheimer’s disease, identifying acylcarnitines and amino acids as deeply tied to disease progression. Specific target proteins related to acylcarnitines were identified, and the clusters or molecular subtypes identified correlated with disease severity and cognitive dysfunction.
Clustering patients based on their metabolic profile has become a popular approach, especially for complex diseases where a single diagnostic label can include numerous sub-pathologies. For example, I discuss the use of such grouping techniques to study asthma in the field of epidemiology with Rachel Kelly on another episode of The Metabolomist. In a 2021 paper, Kelly and her team identified clinically meaningful endotypes of asthma that not only helped identify more refined pathophysiologies than with clinical measures of the disease, but also provided a tool to group patients for further personalized treatments (Kelly et al. 2021).
Treatment optimization
Personalized treatment is of course a priority in precision medicine. More targeted treatments increase efficacy, often through lower doses, while reducing side effects. The one-size-fits-all approach that was once the holy grail of pharmaceutical development has become increasingly unworkable, especially in the context of chronic disease where multiple mechanisms may lead to the same diagnosis.
Returning to ground zero, incorporating sex as a biological variable in clinical trials and treatment planning has been used to optimize immunotherapy strategies for both women and men. A systematic review and meta-analysis of randomized controlled trials of immune checkpoint inhibitors (ICI) with sex-disaggregated results found that while ICI can improve survival for patients with advanced melanoma or non-small-cell lung cancer, the magnitude of benefit is sex-dependent (Conforti et al. 2018). The authors strongly recommend the inclusion of women in clinical trials – a practice still not consistently applied. In both the US and Europe, pre-menopausal women were actively excluded from most clinical trials until the 1990s. After that, the inclusion of women was not precluded, but was not mandatory either. Today, however, reporting sex distributions and sex-disaggregated results is increasingly seen as essential in clinical trials.
Nutritional guidance
The field of nutrition science was among the first to embrace metabolomics. What we eat is clearly a direct provider of metabolites that are absorbed through our digestive tract, but our food also has an impact on our metabolism and health (Limonciel et al. 2013). In addition, our metabolome reflects our metabolism, which deeply impacts how we process and respond to food.
Metabotyping, or classifying individuals into metabolic phenotypes, is a powerful tool in precision nutrition (Hillesheim et al. 2020). These “metabotypes” can enhance personalized nutrition by identifying subgroups that respond differently to dietary interventions, thereby improving health outcomes and informing targeted nutritional strategies. For example, in a 2023 study, metabotypes were used to tailor personalized nutrition advice, resulting in improved dietary quality and reduced plasma cholesterol levels compared to generic dietary recommendations (Hillesheim et al. 2023).
Outlook
Metabolomics offers a wealth of information to support a precision medicine approach. By analyzing broad panels of metabolites, we gain deep insights into an individual’s biochemical state, capturing real-time, systems-level information that reflects genetics, environment, lifestyle, nutrition and disease status. This comprehensive snapshot allows us to detect subtle shifts in interconnected pathways and identify molecular signatures unique to patient subgroups or even individuals.
To use this tool effectively, careful study design is crucial to control for confounding variables, and expert interpretation is essential to translate complex data into meaningful insights. Unsupervised analyses of large datasets are a wonderful source of original findings, but the final interpretation to leverage the data must be done considering our current knowledge of metabolism and medicine, as I explain in my book.
Tools like MetaboINDICATOR, which catalog disease-associated metabolic shifts and compute biologically relevant sums and ratios, provide valuable pre-interpretation layers that bridge data and decision-making. These insights form the foundation for developing personalized diagnostics, prognostics, therapeutic and nutritional strategies.
In the end, metabolomics doesn’t just deepen our understanding of disease, it enables us to tailor interventions to the individual and deliver truly personalized, precise healthcare. It’s an exciting time to work in science!
Sign up for our newsletter to be notified when the next blog on 5P medicine comes out.
References
Arnold et al.: Sex and APOE ε4 genotype modify the Alzheimer’s disease serum metabolome (2020) Nature | https://doi.org/10.1038/s41467-020-14959-w
Conforti et al.: Cancer immunotherapy efficacy and patients’ sex: a systematic review and meta-analysis (2018) Lancet | https://doi.org/10.1016/S1470-2045(18)30261-4
Hillesheim et al.: Metabotyping and its role in nutrition research (2020) Cambridge | https://doi.org/10.1017/S0954422419000179
Hillesheim et al.: Using a metabotype framework to deliver personalized nutrition improves dietary quality and metabolic health parameters: A 12-week randomized controlled trial (2023) Wiley | https://doi.org/10.1002/mnfr.202200620
Horgusluoglu et al.: Integrative metabolomics-genomics approach reveals key metabolic pathways and regulators of Alzheimer’s disease (2021) Alzheimer | http://doi.org/10.1002/alz.12468
Kelly et al.: Metabo-endotypes of asthma reveal differences in lung function: Discovery and validation in two TOPMed cohorts (2021) ATS | http://doi.org/10.1164/rccm.202105-1268OC
Pietzner et al.: Plasma proteome and metabolome characterization of an experimental human thyrotoxicosis model (2017) BMC Medicine | http://doi.org/10.1186/s12916-016-0770-8
Wishart et al.: Emerging applications of metabolomics in drug discovery and precision medicine (2016) Nature | https://doi.org/10.1038/nrd.2016.32


