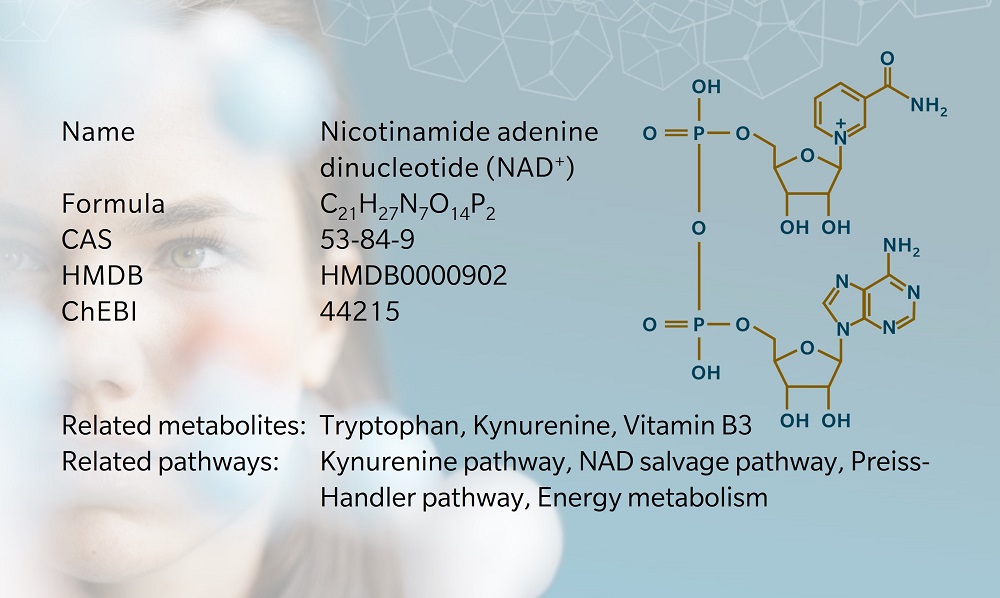
- History & evolution
- Biosynthesis vs. dietary uptake
- NAD+ and its role in metabolism
- NAD+ and ageing
- NAD+ and cancer
- NAD+ and cardiometabolic disease
For this article, we welcome as a guest author Sofia Moco of the Vrije Universiteit Amsterdam who kindly contributed her expertise on her favorite metabolite.
History & Evolution
1906 role in fermentation described by Harden and Young | 1920s -1930s structure elucidation | 1960s discovery of signaling role
NAD+ was initially described as a ‘coferment’ by Arthur Harden and William John Young, who discovered that it accelerated the rate of alcoholic fermentation when they were studying yeast fermentation in the early 20th century (Harden and Young 1906).
Hans von Euler-Chelpin would later identify important chemical features of NAD+, such as the presence of a sugar, adenine and phosphate (Euler 1929).
Von Euler-Chelpin and Harden shared the Nobel Prize of Chemistry in 1929 for “their investigations on the fermentation of sugar and fermentative enzymes”, which included their pioneering work on what they coined ‘cozymase’ (NAD+).
In his acceptance speech, von Euler-Chelpin said ‘…cozymase is one of the most widespread and biologically most important activators within the plant and animal world’ (Euler 1929). In 1936, another Nobel Prize laureate, Otto Heinrich Warburg, identified the pyridine ring as the hydrogen-transferring moiety of NAD+ (Warburg and Christian 1936). This was later shown to play a critical role in redox reactions and metabolism.
The structural features of NAD+ were uncovered over the years through a combination of available techniques, including elemental analysis, enzymology, ultraviolet absorbance, isotopes and mass spectrometry (LePage 1947; Mauzerall and Westheimer 1955; Pullman et al. 1954; Siegel et al. 1959).
The first nuclear magnetic resonance spectra of diphosphopyridine nucleotide (DNP, a former name given to NAD+) and its derivatives were recorded in the 1960s, using a 40 MHz spectrometer (Jardetzky and Wade-Jardetzky 1966; Jardetzky et al. 1963).
Nicotinamide adenine dinucleotide is found in virtually all living cells in the micromolar range. Levels of NAD+ are lower in the cytosol than in the nucleus and mitochondria (Xie et al. 2020). The NAD+/NADH ratio greatly differs according to cell type and condition (NADH being the reduced form of NAD+).
Whilst NAD+ is an indispensable metabolite, the biosynthetic turnover is likely to differ in different tissues, as observed in animal studies (Liu et al. 2018).
Biosynthesis vs. dietary uptake
While NAD+ is not directly absorbed from food, it can be synthesized via three main pathways in humans (Giner et al. 2021):
- the Preiss-Handler pathway with niacin as precursor
- the salvage pathway with nicotinamide as precursor
- the de novo pathway with tryptophan as precursor.
Both niacin and nicotinamide belong to the vitamin B3 family that was discovered as a precursor of NAD+ in the late 1930s (Elvehjem et al. 1974). Humans source vitamin B3 from food, with the dietary recommendation in both EU and USA set at 1.3–1.6 mg per megajoule.
Niacin is found in high amounts in fish and meat (e.g. around 55 mg per 100 g in tuna), but also in many plant-based foods, including coffee (USDA 2022).
Vitamin B3 deficiency is generally associated with malnutrition and causes pellagra, first identified as a skin condition in 1735. The symptoms of pellagra are described by the 4Ds: diarrhea, dermatitis, dementia and death. Pellagra became more prevalent as consumption of maize, which is low in niacin, increased (Cantó et al. 2015).
Aztecs and Mayans would pre-treat maize with alkali, which increased niacin bioavailability, made the corn easier to handle and removed mycotoxins. A diet rich in untreated maize can easily lead to pellagra due to lack of nutrients.
Another precursor of NAD+ is tryptophan, an essential amino acid found in a variety of protein-rich dietary sources. This was discovered through a nutritional study, where the amino acid was shown to be capable of replacing niacin in vitamin B3-deficient individuals (Krehl et al. 1945). De novo NAD+ synthesis occurs via the kynurenine pathway, with quinolinic acid as an intermediate.
Given its lengthy metabolic path towards nicotinamide adenine dinucleotide and the multiple routes that can divert tryptophan from NAD+ production, tryptophan is a less prominent precursor of NAD+.
While different precursors lead to increased levels of NAD+, each precursor has a unique pharmaco-kinetic and dynamic profile, which in turn influences its bio-efficacy. They also produce and excrete different sets of catabolites.
Endogenously occurring nicotinamide riboside (NR), nicotinamide mononucleotide (NMN), and their recently discovered reduced forms (NRH and NMNH), have been explored as pharmacological precursors (Giner et al. 2021).
NAD+ and its role in metabolism
NAD+ is best known as a cofactor that enables redox reactions, but it also acts as a signaling molecule and as a substrate in post-translational modification of proteins.
The oxidized form (NAD+) and reduced form (NADH) make up a coenzyme pair that participates in hundreds of metabolic reactions, acting as a cofactor in redox reactions. NAD+/NADH have crucial roles in central metabolic processes essential in the production of cellular energy.
In glycolysis and in the tricarboxylic acid (TCA) cycle, NAD+ is reduced to NADH, which provides protons to the mitochondrial respiratory chain for oxidative phosphorylation, by which adenosine 5′-triphosphate (ATP) is generated.
The discovery of the non-redox role of NAD+ dates back to the 1960s. nicotinamide adenine dinucleotide was identified as a mediator of ADP-ribosylation, a type of post-translational modification of proteins involved in processes such as cell signaling, gene expression, cellular repair, proteostasis and circadian rhythm (Chambon et al. 1963).
Here, NAD+ serves as a substrate for enzymes such as sirtuins, poly (ADP-ribose) polymerases (PARPs), sterile alpha and toll/interleukin receptor motif-containing protein (SARM) and ADP-ribosyl cyclases (Giner et al. 2021).
Owing to dissociation constants for NAD+ near physiological concentration, these enzymes sense NAD+ levels and adapt their downstream signaling accordingly when NAD+ levels drop, thus conferring a crucial regulatory role to nicotinamide adenine dinucleotide.
NAD+ levels can drop due to a decrease in the NAD+/NADH ratio (NADH is not oxidized back to NAD+) or because the overall NAD concentration decreases in the cell.
NAD+ and ageing
NAD+ levels decrease with age. In aging muscle, NAD+ levels are depleted compared to younger individuals (Janssens et al. 2022). This progressive erosion of NAD+ is associated with age-related diseases such as neurodegenerative diseases and cancers. Reciprocally, restoration of NAD+ metabolism and levels has shown positive effects on the same pathologies, as reviewed by Yaku et al. (2018).
This drop in NAD+ levels directly impacts the activity of enzymes like sirtuins and PARPs. Interestingly, this decrease also coincides with an increase in expression and activity of CD38, a NAD+ degradation enzyme, or NADase (Camacho-Pereira et al. 2016).
CD38 is an enzyme involved in immune response and energy metabolism that converts NAD+ to nicotinic acid adenine dinucleotide phosphate (NAADP), a second messenger regulating intracellular calcium release.
Upon CD38 inhibition and in knockout animals, nicotinamide adenine dinucleotide levels are higher than in wild type and improve glucose and lipid homeostasis (Schultz and Sinclair 2016; Escande et al. 2013).
While ageing is overall a natural process, there are factors that can accelerate it. Thus, biological age and actual age don’t always match. Since NAD+ levels associate with several age-related diseases, this brings about the question: is this NAD+ decrease a cause or a consequence of (accelerated) ageing?
Anti-ageing therapies aimed at increasing NAD+ levels suggest that it is at least a factor that can be modulated. Interestingly, lifestyle changes that reduce the energy load such as fasting, glucose deprivation, dietary restriction and exercise, typically increase the levels of oxidized NAD+.
Inversely, Western-style diet (high fat, high sugar) decreases nicotinamide adenine dinucleotide levels and accelerates ageing (reviewed by Campisi et al. (2019)). NAD+ is also linked to several molecular hallmarks of ageing, including inflammation and mitochondrial dysfunction, via its role as a substrate for other inflammation-related enzymes and sirtuins (Xie et al. 2020).
NAD+ and cancer
NAD(H) is essential for the respiratory chain of mitochondria where ATP is generated via oxidative phosphorylation. Thus, a well-functioning NAD system is essential for cells with high energy demands, such as highly proliferative cancer cells. This has prompted the search for targeted approaches aimed at lowering NAD+ levels in cancer cells.
Since much of the newly synthetized nicotinamide adenine dinucleotide comes from the salvage pathway, the inhibition of the enzyme nicotinamide phosphoribosyltransferase (NAMPT) has been a therapeutic target of choice, albeit without much efficacy in the clinics so far (Ghanem et al. 2021). Even if the therapy targets cancer cells, NAD+ does not act solely as a redox cofactor; it is also a substrate for protective mechanisms against cancer.
For example, PARP enzymes consume the NAD+ generated by NAMPT in the salvage pathway to support DNA repair mechanisms. Thus, when the pathway is blocked and NAD+ levels drop, DNA damage is more difficult to control and mutations are more likely to arise (Xie et al. 2020; Ghanem et al. 2021).
NAD+ and cardiometabolic disease
A decrease in NAD+/NADH ratio has been associated with diabetes (Song et al. 2019). This situation, termed “pseudohypoxia”, occurs in both type 1 and type 2 diabetes animal models via different mechanisms (Fan et al. 2020).
In each case, NADH is not oxidized to NAD+, leading to a drop in NAD+ levels and NADH accumulation. This results in redox imbalance and reductive stress, leading to oxidative stress and cellular damage over time. Interestingly, this seems to occur in the cytosol but not in mitochondria.
Pseudohypoxia has been described in numerous organs of diabetic animals models, including the liver, eyes, kidneys, pancreas, skeletal muscle, nerves and adipose tissue (Fan et al. 2020; Williamson et al. 1993).
In the heart, nicotinamide adenine dinucleotide imbalance exacerbated diabetic cardiopathy in a diabetic mouse model (Chiao et al. 2021). Another characteristic of pseudohypoxia in diabetes is an impairment of the salvage pathway further contributing to the lack of NAD+ (Fan et al. 2020).
References
Camacho-Pereira et al.: CD38 Dictates Age-Related NAD Decline and Mitochondrial Dysfunction through an SIRT3-Dependent Mechanism (2016) Cell metabolism | http://doi.org/10.1016/j.cmet.2016.05.006
Campisi et al.: From discoveries in ageing research to therapeutics for healthy ageing (2019) Nature | http://doi.org/10.1038/s41586-019-1365-2
Cantó et al.: NAD(+) Metabolism and the Control of Energy Homeostasis: A Balancing Act between Mitochondria and the Nucleus (2015) Cell metabolism | http://doi.org/10.1016/j.cmet.2015.05.023
Chambon et al.: Nicotinamide mononucleotide activation of a new DNA-dependent polyadenylic acid synthesizing nuclear enzyme (1963) Research Communications | http://doi.org/10.1016/0006-291X(63)90024-X
Chiao et al.: NAD+ Redox Imbalance in the Heart Exacerbates Diabetic Cardiomyopathy (2021) Circulation. Heart failure | http://doi.org/10.1161/CIRCHEARTFAILURE.120.008170
Elvehjem et al.: The isolation and identification of the anti-black tongue factor (1974) Nutrition reviews | http://doi.org/10.1111/j.1753-4887.1974.tb06263.x
Escande et al.: Flavonoid apigenin is an inhibitor of the NAD+ ase CD38: implications for cellular NAD+ metabolism, protein acetylation, and treatment of metabolic syndrome (2013) Diabetes | http://doi.org/10.2337/db12-1139
Euler et al.: Fermentation of Sugars and Fermentative Enzymes (1929) | https://www.nobelprize.org/prizes/chemistry/1929/euler-chelpin/lecture
Fan et al.: Impaired nicotinamide adenine dinucleotide (NAD+ ) metabolism in diabetes and diabetic tissues: Implications for nicotinamide-related compound treatment. (2020) Journal of Diabetes Investigation | http://doi.org/10.1111/jdi.13303
Ghanem et al.: Advances in NAD-Lowering Agents for Cancer Treatment (2021) Nutrients | http://doi.org/10.3390/nu13051665
Giner et al.: A Method to Monitor the NAD+ Metabolome-From Mechanistic to Clinical Applications (2021) International Journal of Molecular Sciences | http://doi.org/10.3390/ijms221910598
Harden et al.: The Alcoholic Ferment of Yeast-Juice (1906) Proceedings of the Royal Society of London Series | https://doi.org/10.1098/rspb.1906.0029
Janssens et al.: Healthy aging and muscle function are positively associated with NAD+ abundance in humans (2022) Nat Aging | http://doi.org/10.1038/s43587-022-00174-3
Jardetzky et al.: The Conformation of Pyridine Dinucleotides in Solution (1966) Journal of Biological Chemistry | http://doi.org/10.1016/S0021-9258(18)96961-9
Jardetzky et al.: Proton magnetic resonance investigation of enzyme-coenzyme complexes (1963) Nature | http://doi.org/10.1038/197183a0.
Krehl et al.: Growth-Retarding Effect Of Corn In Nicotinic Acid-Low Rations And Its Counteraction By Tryptophane (1945) Science (New York, N.Y.) | http://doi.org/10.1126/science.101.2628.489 .
LePage et al.: Preparation Of Diphosphopyridine Nucleotide. In Journal of Biological Chemistry (1947) Journal of Biological Chemistry | http://doi.org/10.1016/S0021-9258(17)30921-3
Liu et al.: Quantitative Analysis of NAD Synthesis-Breakdown Fluxes. (2018) Cell metabolism | http://doi.org/10.1016/j.cmet.2018.03.018
Mauzerall et al.: 1-Benzyldihydronicotinamide—A Model for Reduced DPN. (1955) J. Am. Chem. Soc. | http://doi.org/10.1021/ja01613a070
Pullmann et al.: (1954): On the structure of reduced diphosphopyridine nucleotide. (1954) Journal of Biological Chemistry | https://pubmed.ncbi.nlm.nih.gov/13130534/
Schultz et al.: Why NAD(+) Declines during Aging: It’s Destroyed. (2016) Cell metabolism| http://doi.org/10.1016/j.cmet.2016.05.022
Siegel et al.: Ultraviolet absorption spectra of DPN and analogs of DPN. (1959) Archives of Biochemistry and Biophysics | http://doi.org/10.1016/0003-9861(59)90124-9
Song et al.: Role of pseudohypoxia in the pathogenesis of type 2 diabetes. (2019) Hypoxia | http://doi.org/10.2147/HP.S202775
USDA: Food and Nutrient Database for Dietary Studies (FNDDS) | Ag Data Commons. (2022) https://data.nal.usda.gov/dataset/food-and-nutrient-database-dietary-studies-fndds
Warburg et al.: Pyridin, der wasserstoffübertragende Bestandteil von Gärungsfermenten. (1936) HCA | http://doi.org/10.1002/hlca.193601901199
Williamson et al.: Hyperglycemic pseudohypoxia and diabetic complications. (1993) Diabetes | http://doi.org/10.2337/diab.42.6.801
Xie et al.: NAD+ metabolism: pathophysiologic mechanisms and therapeutic potential. (2020) Sig Transduct Target Ther | http://doi.org/10.1038/s41392-020-00311-7
Liu et al.: Quantitative Analysis of NAD Synthesis-Breakdown Fluxes (2018) Cell metabolism | http://doi.org/10.1016/j.cmet.2018.03.018
Mauzerall et al.: 1-Benzyldihydronicotinamide—A Model for Reduced DPN (1955) J. Am. Chem. Soc. | http://doi.org/10.1021/ja01613a070
Pullman et al.: On the structure of reduced diphosphopyridine nucleotide (1954) Journal of Biological Chemistry | Available online at https://pubmed.ncbi.nlm.nih.gov/13130534/
Schultz et al.: Why NAD(+) Declines during Aging: It’s Destroyed (2016) Cell metabolism | http://doi.org/10.1016/j.cmet.2016.05.022 .
Siegel et al.: Ultraviolet absorption spectra of DPN and analogs of DPN (1959) Archives of Biochemistry and Biophysics | http://doi.org/10.1016/0003-9861(59)90124-9
Song et al.: Role of pseudohypoxia in the pathogenesis of type 2 diabetes (2019) Hypoxia | http://doi.org/10.2147/HP.S202775 .
USDA : Food and Nutrient Database for Dietary Studies (FNDDS) (2022) Ag Data Commons | https://data.nal.usda.gov/dataset/food-and-nutrient-database-dietary-studies-fndds
Warburg et al.: Pyridin, der wasserstoffübertragende Bestandteil von Gärungsfermenten (1936) Helvetica Chimica Acta | http://doi.org/10.1002/hlca.193601901199
Williamson et al.: Hyperglycemic pseudohypoxia and diabetic complications (1993) Diabetes | http://doi.org/10.2337/diab.42.6.801
Xie et al.: NAD+ metabolism: pathophysiologic mechanisms and therapeutic potential (2020) Sig Transduct Target Ther | http://doi.org/10.1038/s41392-020-00311-7
Yaku et al.: NAD metabolism: Implications in aging and longevity (2018) Ageing research reviews | http://doi.org/10.1016/j.arr.2018.05.006