- Foreword
- How metabolomics is improving healthcare: 6 must-read studies from 2025
- Hepatology: functional detox capacity beyond fibrosis
- Cardiometabolic health: anticipating disease before symptoms
- Cohorts and mGWAS: from genetic signals to functional meaning
- Oncology: detecting cancer before its manifestation
- Neuropsychiatry and microbiome: modulating behavior through microbial metabolism
- Nanomedicine: designing safer therapies through metabolomics & lipidomics
- The next steps for metabolomics in 5P medicine
Foreword by Alice Limonciel, Chief Scientific Officer at biocrates
After decades of development, we are on the cusp of integrating metabolomics into medical practice. Numerous examples already exist in clinical settings, the result of the dedicated labor of passionate scientists and clinicians who recognized the potential of this omic and applied it across all areas of medicine. However, the broad adoption of metabolomics on a scale comparable to what we now see with genomics requires the development of robust, transferable, and scalable technology, which has been the mission of biocrates for the past 20 years.
In 2025, we chose to showcase the wide-ranging potential of metabolomics for all aspects of 5P medicine, from preventing chronic disease in a single individual through personalized strategies to enabling multiomic analyses in large cohort studies.
For this article, Franziska Hörburger selected six studies published in 2025 by biocrates’ community of users. These examples pave the way for the imminent implementation of metabolomics beyond the research lab and into clinical practice and our everyday lives. They span multiple regions, therapeutic areas, and dimensions of the future implementation of metabolomics in medicine and drug development.
These scientists are part of a community of early adopters of metabolomics, a technology that will transform how we understand and practice medicine. May this article inspire you and your team to join this community in 2026!
How metabolomics is improving healthcare: 6 must read studies from 2025
5P medicine – preventive, predictive, precision, population-based, and participatory – represents a paradigm shift in healthcare. It moves away from reactive treatment toward proactive, patient-centric strategies built on molecular insights. At its core, 5P medicine leverages high-quality standardized technologies to capture biology in unprecedented detail. It enables clinicians and researchers to predict disease risk, personalize interventions, while engaging patients in their individual health journey and at the population scale.
Among the molecular technologies shaping modern medicine, metabolomics stands out. While genomics can predict disease risk, it offers only a static view, like a snapshot of predisposition. Metabolomics, in contrast, captures the biochemical fingerprints of life, reflecting the dynamic interplay of genes, environment, lifestyle, microbiome, and pharmacological influences. This real-time perspective makes metabolomics indispensable for understanding complex chronic diseases, where genetic information alone cannot explain onset, progression, or therapeutic response.
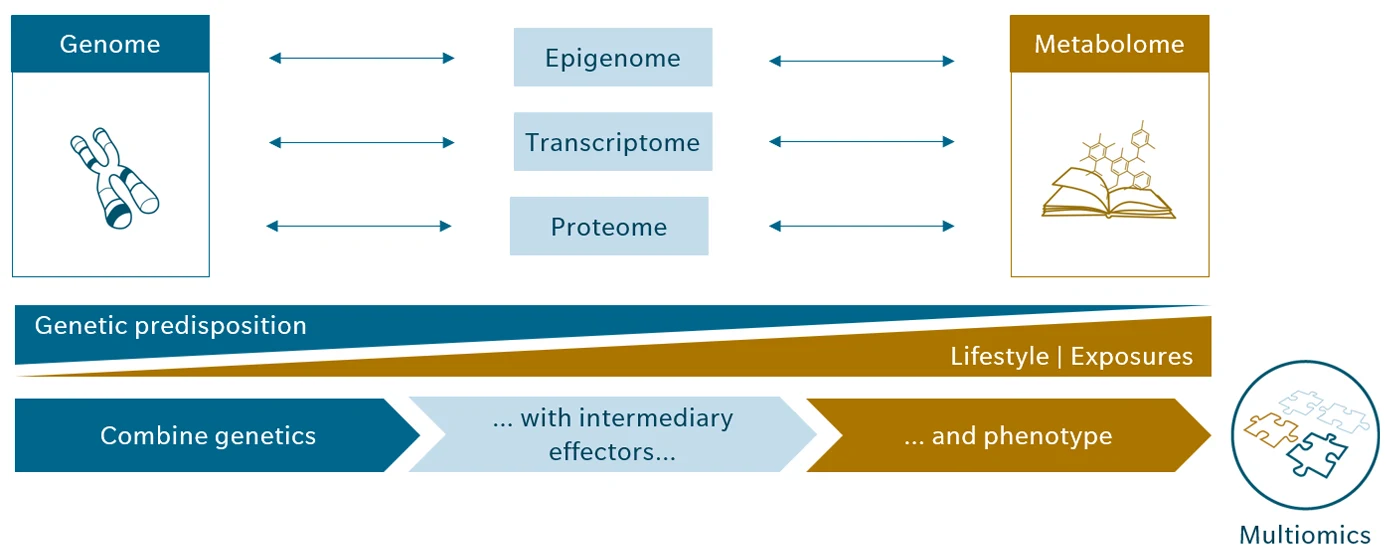
Figure 1: Molecular health beyond genetic predisposition
When metabolomics is combined with other omics technologies, such as genomics or proteomics, the picture becomes even richer. Proteomics adds information about enzyme abundance and signaling networks, complementing metabolomics’ readout of pathway activity and flux. Together, these layers create a detailed system-level view of health and pathology, connecting genetic predisposition to molecular function and clinical phenotype. This integrated approach transforms omics from isolated data streams into actionable insights, connecting molecular complexity and medical decision-making based on the 5P concept.
Applying metabolomics within the 5P framework can be summarized in three steps. First, screen samples using broad metabolomics and lipidomics profiling. Second, leverage data to uncover biological meaning. This involves interpreting metabolite patterns, sums, and ratios, and linking them to pathways and literature. Third, translate insights into solutions: providing predictive biomarkers, metabotypes, risk scores, and decision-support tools that transform medicine from reactive to proactive.
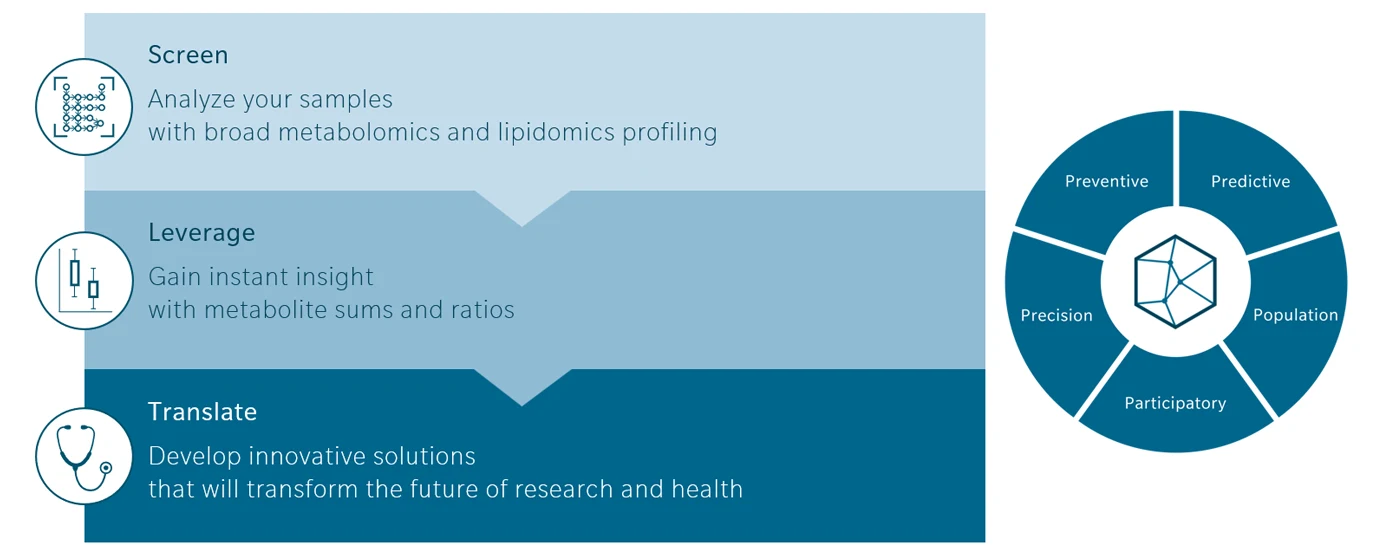
Figure 2: Workflow for applying metabolomics in the 5P framework
This vision is not theoretical; it is already happening. Across biological matrices, continents and disciplines, researchers and clinicians are using biocrates’ technology to deliver actionable insights in fields as varied as hepatology, oncology, neuropsychiatry, cardiometabolic health, population studies, and nanomedicine.
Here we review six publications from 2025 in high-impact journals that illustrate how one standardized platform can drive breakthroughs aligned with the principles of 5P medicine.
Hepatology: functional detox capacity beyond fibrosis
Sugimoto et al.: Hepatic stellate cells control liver zonation, size and functions via R-spondin 3. Nature (2025), 640(8059):752–761 | https://doi.org/10.1038/s41586-025-08677-w Figure under creative commons license CC BY 4.0.
Sugimoto and colleagues uncovered how hepatic stellate cells orchestrate liver zonation and detoxification through the signaling molecule R-spondin 3 (RSPO3), a key regulator of the WNT pathway. When RSPO3 is lost, hepatocyte zonation collapses and regenerative capacity declines. Beyond structural changes, RSPO3 profoundly influences detoxification by modulating cytochrome P450 activity, which in turn alters circulating metabolite profiles. Liver tissue of RSPO3-deficient mice featured striking shifts in bile acid composition, particularly taurocholic, tauromuricholic, and taurochenodeoxycholic acids.
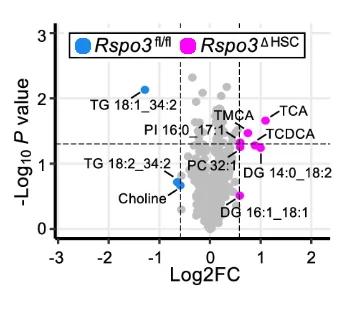
Furthermore, changes in steroid metabolism, lipid oxidation, and xenobiotic accumulation have been revealed. These metabolomic signatures predict functional liver capacity, drug metabolism potential, and ultimately toxicity risk. By identifying RSPO3 as both a prognostic and mechanistic marker, this work opens the door to early intervention, personalized risk stratification, and tailored therapeutic approaches for liver fibrosis, particularly in alcoholic liver disease and metabolic dysfunction associated liver disease.
Cardiometabolic health: anticipating disease before symptoms

Zieleniewska et al.: Preclinical Atherosclerosis and Prediabetes: A Cross-Sectional Metabolic Assessment In Apparently Healthy Population. Cardiovascular Diabetology (2025), 24(1), 280 | https://doi.org/10.1186/s12933-025-02841-2 Figure under creative commons license CC BY-NC-ND 4.0.
Cardiovascular disease and diabetes often develop silently over years, making early detection critical. The metabolic foundation of preclinical atherosclerosis compared to prediabetes was explored in 447 participants from the Bialystok PLUS cohort.
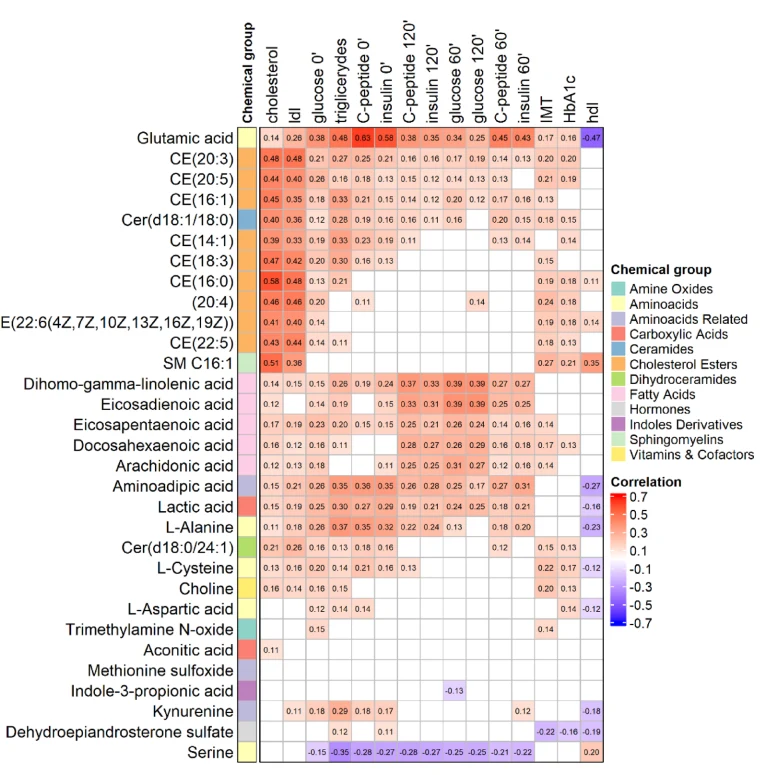
The analysis uncovered distinct and shared metabolic signatures in plasma for both conditions. Prediabetes exerted a broader impact on amino acid metabolism, lipid signaling and enzymatic activities than atherosclerosis. Glutamic acid, lactic acid, and alanine were strongly associated with prediabetes, indicating dysglycemia. Atherosclerosis was linked to lipid remodeling patterns captured by MetaboINDICATORs, including the ratio of polyunsaturated (PUFA)-lysophosphatidylcholines versus saturated fatty acids, the sum of steroid hormones, and cholesteryl ester (CE) classes such as monounsaturated CEs and long-chain fatty acids CEs. Trimethylamine N-oxide (TMAO) emerged as a unique link between prediabetes and its interaction with vascular pathology. At the same time, glutaminase activity, assessed through the glutamate/glutamine ratio, stood out as a robust shared predictor of both conditions. Metabolite set enrichment analysis observed converging disturbances in glutathione and folate metabolism, mitochondrial function, redox regulation and inflammation.
Cohorts and mGWAS: from genetic signals to functional meaning

Kodate et al.: Simulating metabolic pathways to enhance interpretations of metabolome genome-wide association studies. Scientific Reports (2025), 15(1), 17035 | https://doi.org/10.1038/s41598-025-01634-7 Figure under creative commons license CC BY-NC-ND 4.0.
Metabolome-genome-wide association studies (mGWAS) link genetic variation to metabolite concentrations in large cohorts. It provides great predictive power of risk models and enables rational intervention based on individual metabolic architecture. However, this powerful approach has some limitations: observed associations may reflect indirect effects through unmeasured metabolites, and the biological significance of many variants remains uncertain. To overcome these challenges, Kodate and colleagues combined mGWAS with mechanistic metabolic simulations, creating a framework that moves beyond correlation to causation.
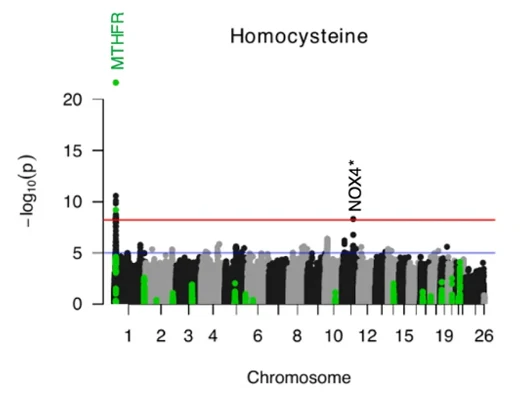
By systematically adjusting enzyme reaction rates to mimic genetic variants, the team simulated their impact on plasma metabolite levels and validated most variant-metabolite pairs identified by mGWAS. For example, homocysteine was confirmed as a metabolite strongly influenced by methylenetetrahydrofolate reductase (MTHFR) activity. Both mGWAS and simulation agreed that reduced MTHFR activity increases homocysteine levels, reinforcing its role in folate and methionine metabolism. These simulations also revealed additional fluctuations that mGWAS had missed, suggesting that some associations could gain significance with larger sample sizes. Importantly, the study categorized enzymes into three tiers based on their influence on metabolite concentrations, highlighting variants with minimal biological impact and prioritizing those with strong functional relevance. This distinction is critical for guiding preventive strategies and therapeutic development.
Oncology: detecting cancer before its manifestation

Schulze et al.: Metabolomic liquid biopsy dynamics predict early-stage HCC and actionable candidates of human hepatocarcinogenesis. JHEP Reports (2025), 7(5):101340 | https://doi.org/10.1016/j.jhepr.2025.101340. Figure under creative commons license CC BY 4.0.
Hepatocellular carcinoma (HCC) develops through progressive metabolic reprogramming that begins long before tumors become radiologically or clinically detectable. In a global cohort of 654 patients, serum metabolome profiling captured these early, system-level alterations and predicted malignant transformation before overt tumor manifestation.
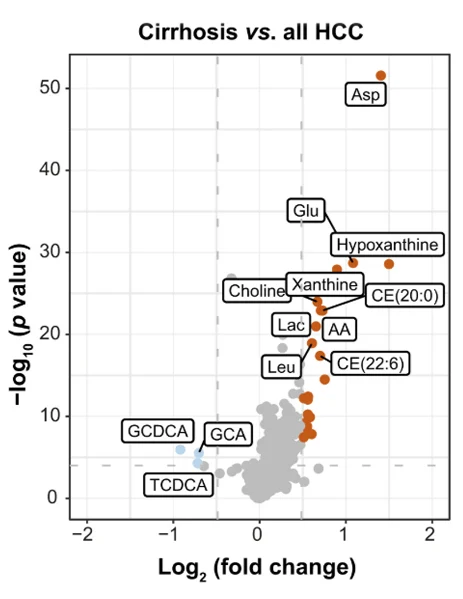
Across chronic liver disease, cirrhosis, initial HCC, and advanced HCC, amino acid-, lipid-, and nucleotide-related pathways were systematically deregulated, with aspartic acid, glutamic acid, taurine, and hypoxanthine emerging as key markers. In a phase II biomarker case-control study, a blood-based metabolite signature achieved an area under the curve (AUC) of 94% for distinguishing early-stage HCC from cirrhotic controls, with independent validation in an external cohort. Multiomics integration links these circulating markers to enzymatic nodes such as RRM2, GMPS, and BCAT1 – targets for precision oncology. By providing a validated, minimal-invasive liquid biopsy that outperforms current surveillance tools, serum metabolomics enables predictive identification of cancer risk, chemoprevention strategies, and personalized monitoring.
Neuropsychiatry and microbiome: modulating behavior through microbial metabolism

Park et al.: Gut microbiota and brain-resident CD4+ T cells shape behavioral outcomes in autism spectrum disorder. Nature Communications (2025), 16(1), 1–17 | https://doi.org/10.1038/s41467-025-61544-0 Figure under creative commons license CC BY 4.0.
Autism spectrum disorder (ASD) emerges from complex interactions between neurodevelopment, immune regulation, and the gut microbiome. Metabolites serve as critical messengers of this gut-immune-brain axis, influencing neuroinflammation and neurotransmitter flux. In a recent study, the absence of gut microbiota in male mice ameliorated ASD-associated behaviors and reduced inflammatory brain-resident CD4⁺ T cells, while depletion of these T cells further mitigated neuroinflammation and behavioral abnormalities. Fecal metabolomics in a mouse model of ASD revealed several microbial and metabolic regulators of ASD, particularly those affecting the glutamate/gamma-amino-butyric acid (GABA) ratio and neurotoxic intermediates such as 3-hydroxyglutaric acid.
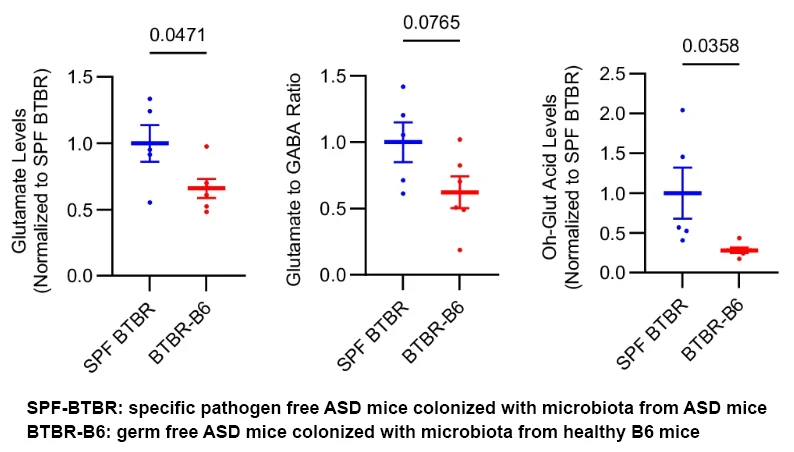
While GABA levels remained stable, the glutamate/GABA ratio was significantly elevated in ASD mice treated with a broad-spectrum antibiotic cocktail (vancomycin, neomycin, metronidazole), a group that also showed enrichment of Lactobacillus species compared to neurotypical controls. Strikingly, beneficial microbiota, derived from healthy mice or administered as probiotics, reversed this imbalance. These findings underscore how metabolites from live bacteria can drive or mitigate ASD-like behaviors by altering excitatory/inhibitory signaling and immune tone. Ultimately, the study demonstrates that gut microbiota can override genetic predisposition in ASD, highlighting a powerful opportunity for metabolomics-informed interventions that rebalance neuroactive metabolites, suppress neuroinflammation, and improve behavioral outcomes.
Nanomedicine: designing safer therapies through metabolomics & lipidomics

Shaw et al.: Inflammatory disease progression shapes nanoparticle biomolecular corona-mediated immune activation profiles. Nature Communications (2025),16(1), 924 | https://doi.org/10.1038/s41467-025-56210-4 Figure under creative commons license CC BY 4.0.
Polymeric nanoparticles (NPs) are engineered to carry, protect, and deliver bioactive molecules or modulate biological responses. Their biological identity, the biomolecular corona, is not fixed by formulation alone but is dynamically shaped by the host environment. Multiomics analysis showed that, during acute systemic inflammation, plasma proteins, lipids, and metabolites change profoundly.
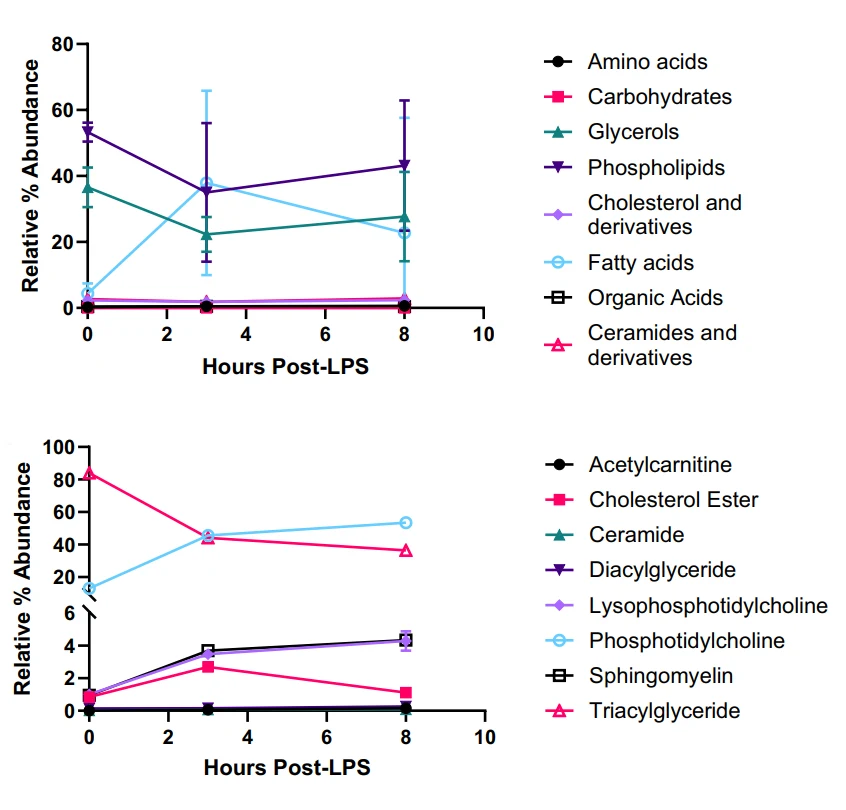
As a result, nanoparticle coronas are reshaped. They feature elevated levels of clotting factors, inflammatory proteins, cytoskeletal components, and lipids such as phosphatidylcholines, sphingomyelins, lysophosphatidylcholines, and fatty acids. These molecular signatures reflect heightened inflammatory activity and trigger immune pathways like TLR4/MyD88/NF-κB. This activation leads to the release of pro-inflammatory cytokines, including TNFα and IL-6. Together, these findings show how metabolic variability determines nanoparticle-based therapeutic efficacy and toxicity risk. The concept of a “personalized biomolecular corona” underscores the need to design nanomedicines that account for patient-specific metabolic states. Incorporating metabolomic profiling into nanoparticle development helps anticipate immune responses, optimize timing, and improve safety.
The next steps for metabolomics in 5P medicine
What unites these global studies performed in various species and matrices beyond their drive to bring medicine to a higher level, is their use of the metabolomics kit technology developed by biocrates.
Across a wide range of applications, our standardized kits provide the reproducible and quality-controlled methods that enable multiomics integration, cohort comparability, and regulatory-compliant workflows.
While 2025 saw the broad application of our MxP® Quant 500 and MxP® Quant 500 XL kits, 2026 will be the year of the MxP® Quant 1000 kit. Our broadest panel to date, this kit expands quantitative analysis to up to 1,233 metabolites from 49 biochemical classes, showing coverage comparable to untargeted metabolomics approaches, yet with the reproducibility and sensitivity of a targeted workflow.
To follow our next steps, make sure to register for our monthly newsletter.
For additional insights also explore a curated selection of 2025 publications from our Biognosys Group partners, Biognosys and PreOmics.


