History & evolution
Biosynthesis vs. dietary uptake
Glycine as a neurotransmitter
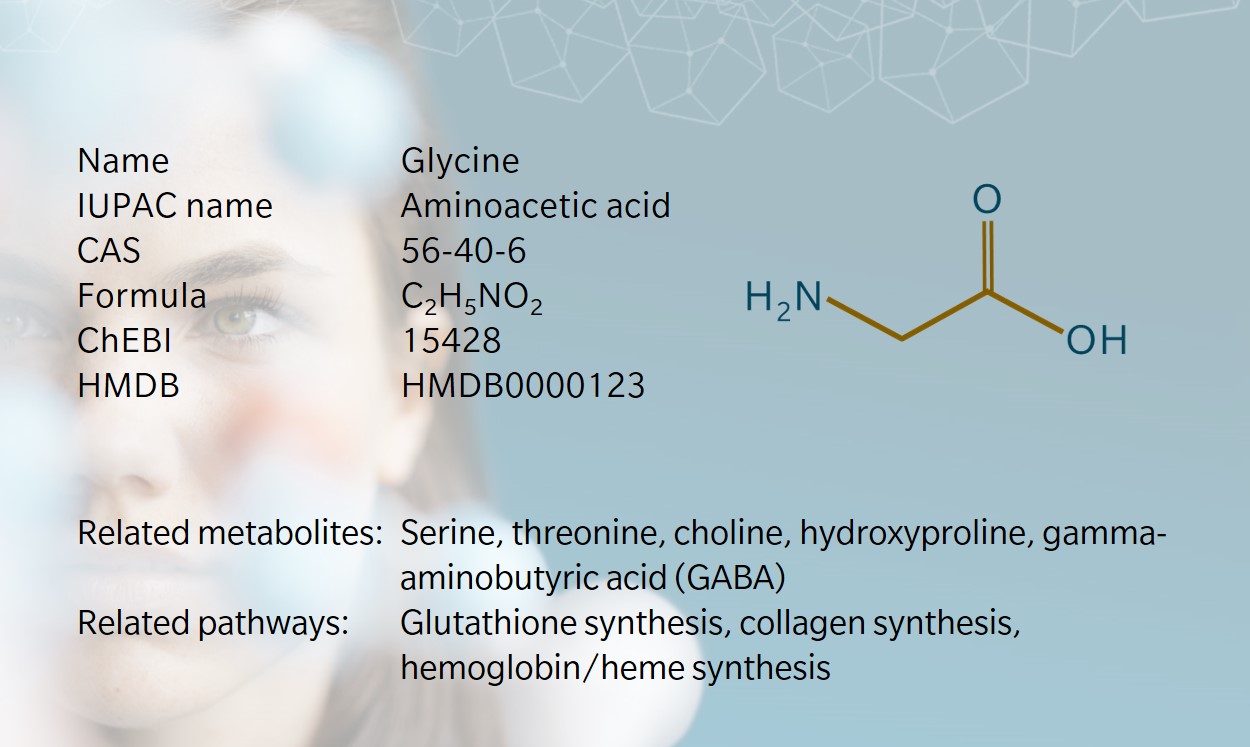
Glycine as a versatile player in metabolic reactions
Glycine and metabolic disorders
Glycine and sleep
History and evolution
1820: discovery (Razak et al. 2017) | 1858: structure identified (Cahours 1858) | 2009: confirmation that glycine is present outside the Earth (Kvenvolden et al.1970)
Glycine is the simplest and smallest amino acid. It was discovered in 1820 by French chemist Henri Braconnot, who boiled gelatin with sulfuric acid (Razak et al. 2017). Braconnot originally called it the “sugar of gelatin,” but Swedish chemist Berzelius later came up with the simpler name “glycine,” derived from the Greek word for “sweet.” Glycine’s structure was first identified in 1858 by Auguste Cahours, who found that glycine was an amine of acetic acid (Cahours 1858).
Interestingly, in 1970, glycine was discovered in the Murchison meteorite (Kvenvolden et al.1970). The presence of glycine outside the Earth was confirmed in 2009, based on samples recovered from the Wild 2 comet by the NASA spacecraft Stardust in 2004. The discovery of glycine in space strengthened the soft-panspermia hypothesis, which proposes that life’s “building blocks” are found all around the universe (Edalati et al. 2022).
In humans, glycine plays a role in metabolic regulation, neurological function, anti-oxidative reactions and protein synthesis. It offers multiple health benefits that may help to treat diabetes, cardiovascular disease, cancer, inflammation and obesity. Glycine is technically a non-essential amino acid, but having too little can impair immune function, growth and nutrient metabolism. It’s therefore considered conditionally essential (Wang et al. 2013).
Biosynthesis vs. dietary uptake
Glycine synthesis is performed in myriad ways primarily in the liver and kidneys (reviewed in Razak et al. 2017). Its best-known precursors include:
- serine and tetrahydrofolate, via the action of serine hydroxy methyltransferase,
- choline or methionine, via the intermediate metabolite sarcosine converted to glycine by sarcosine dehydrogenase
Although glycine is predominantly generated in the liver, it can also be obtained from food. Glycine is found in both animal and plant proteins in the diet, with animal proteins being the most glycine-abundant (Alves et al. 2019). It is a key component of various plant proteins, as in soy and corn. Glycine can also be derived from other foods, such as vegetables, legumes, nuts and dairy products. As is often the case for health, a balanced diet that includes a variety of foods is essential for obtaining optimal quantities of glycine. Glycine absorption occurs in the small intestine, followed by transport to the liver and other organs.
Although glycine can be synthesized in our body, much of it is also consumed in follow-up reactions, and many of the reactions described above can be reversed or are part of a cycle that sees glycine levels only transiently increased. For this reason, parts of our glycine stores need to be sourced from diet, in particular for the synthesis of collagen where glycine is the predominant amino acid (Li et al. 2018).
Glycine as a neurotransmitter
Glycine is both an excitatory and an inhibitory neurotransmitter that is widely distributed in the central nervous system. As an excitatory neurotransmitter, it plays a role in regulating glutamate, helping to maintain neuronal flexibility (López-Corcuera et al. 2001). As an inhibitory neurotransmitter, it modulates neuronal activity by reducing the excitability of cells, binding to specific receptors on their surfaces. Glycine is involved in regulating the activity of many different brain regions, including the spinal cord, hippocampus and cerebellum.
It is believed to play a role in memory formation and learning, controlling pain signals, and processing motor and sensory information. Glycine works by decreasing the amount of calcium that enters the neuron, thereby reducing the likelihood of a neuron firing. It also helps to regulate the production of other neurotransmitters, such as gamma-aminobutyric acid (GABA), which also has an inhibitory effect (López-Corcuera et al. 2001).
Glycine as a versatile player in metabolic reactions
Glycine is used in the liver to synthesize other metabolites, such as bile acids and heme (Garcia-Santos et al. 2017). Glycine is also involved in the synthesis of the tripeptide glutathione (GSH), a powerful antioxidant that helps protect cells from the damaging effects of reactive oxygen species. Additionally, glycine is a precursor for glutamate production, which is also necessary for glutathione synthesis. Therefore, glycine is essential for synthesizing glutathione, and its absence can lead to oxidative damage in cells (McCarty et al. 2018).
Low levels of GSH have been associated with old age and several chronic medical conditions such as hypertension, chronic obstructive pulmonary disease, diabetes and even COVID-19. A recent randomized controlled clinical trial in healthy older adults demonstrated that supplementation with glycine and N-acetylcysteine may support GSH biosynthesis in individuals with increased oxidative stress and compromised GSH stores (Lizzo et al. 2022). These results suggest that adequate glycine uptake or its administration may be essential to maintain health, especially with older age.
In addition to heme and glutathione, glycine is also a primary building block for collagen synthesis. It accounts for around one-third of the amino acids required to form collagen molecules (Li et al. 2018). Collagen is the most abundant protein in the body and is essential to maintain the normal structure and strength of connective tissue, such as skin, bones, cartilage and blood vessels. A lack of glycine impairs collagen formation, and the body may suffer from weakening bones, joints and other connective tissues. In a randomized controlled trial, a spray formulation of collagen precursors, including glycine, was shown to dramatically reduce the severity and duration of oral mucositis (inflammation of the mouth) in patients undergoing hematopoietic stem cell transplantation (Ruggiero et al. 2018).
Glycine and metabolic disorders
Glycine’s involvement in essential metabolic pathways makes it a metabolite of interest in several metabolic disorders. For example, low plasma glycine concentrations have been consistently linked to type 2 diabetes, obesity and non-alcoholic fatty liver disease (Alves et al. 2019). A recently published meta-analysis of metabolomics studies in pre-diabetic and diabetic individuals showed that glycine, together with glutamine, is inversely associated with type 2 diabetes (Guasch-Ferré et al. 2016).
Glycine and sleep
Glycine has been found to have a direct link to sleep (Bannai et al. 2012). Research in rodents suggests that glycine supplementation could lead to improved sleep quality, reduced daytime fatigue, and even enhanced cognitive performance (Kawai et al. 2015). Glycine is thought to induce relaxation and sleepiness by increasing the activity of certain neurotransmitters in the brain, such as serotonin and GABA, both of which regulate sleep.
Highlighting glycine’s role in sleep regulation, non-ketotic hyperglycinemia is a rare metabolic disorder characterized by excessive levels of glycine in the blood, with sleepiness as a main symptom (Ning et al. 2022). Symptoms are usually first seen in newborns, and include poor feeding, weak muscle tone, seizures and coma (Ning et al. 202). Treatment focuses on reducing glycine levels in the blood, through dietary changes and medications (Shelkowitz et al. 2022).
Learn more about the roles of glycine and conjugated bile acids in complex chronic diseases such as cancer, Alzheimer’s disease, depression, inflammatory bowel disease, multiple sclerosis and diabetes in our whitepaper “Complex chronic diseases have a common origin”.
References
Alves A. et al.: Glycine Metabolism and Its Alterations in Obesity and Metabolic Diseases.(2019) Nutrients | https://doi.org/10.3390/nu11061356
Bannai M. et al.: New therapeutic strategy for amino acid medicine: glycine improves the quality of sleep.(2012) Journal of Pharmacological Sciences | https://doi.org/10.1254/jphs.11R04FM
Cahours, A.: Recherches sur les acides aminés [Investigations into aminated acids]. (1858) Comptes Rendus (in French) | Original from University of Minnesota https://babel.hathitrust.org/cgi/pt?id=umn.31951d00008355e&view=1up&seq=1050
Edalati K. et al.: Glycine amino acid transformation under impacts by small solar system bodies, simulated via high-pressure torsion method.(2022) Scientific Reports | https://doi.org/10.1038/s41598-022-09735-3
Garcia-Santos D. et al.: Extracellular glycine is necessary for optimal hemoglobinization of erythroid cells. (2017) Haematologica | https://doi.org/10.3324/haematol.2016.155671
Guasch-Ferré M. et al.: Metabolomics in Prediabetes and Diabetes: A Systematic Review and Meta-analysis (2016) | https://pubmed.ncbi.nlm.nih.gov/27208380/
Kawai N. et al.: The Sleep-Promoting and Hypothermic Effects of Glycine are Mediated by NMDA Receptors in the Suprachiasmatic Nucleus. (2015) Neuropsychopharmacology | https://doi.org/10.1038/npp.2014.326
Kvenvolden K. et al.: Evidence for Extraterrestrial Amino-acids and Hydrocarbons in the Murchison Meteorite. (1970) Nature | https://doi.org/10.1038/228923a0
Li P. et al.: Roles of dietary glycine, proline, and hydroxyproline in collagen synthesis and animal growth (2018) Amino Acids. (2018) | https://doi.org/10.1007/s00726-017-2490-6
Lizzo G. et al.: A Randomized Controlled Clinical Trial in Healthy Older Adults to Determine Efficacy of Glycine and N-Acetylcysteine Supplementation on Glutathione Redox Status and Oxidative Damage. (2022) Front Aging | https://doi.org/10.3389/fragi.2022.852569
López-Corcuera B. et al.: Glycine neurotransmitter transporters: an update. (2001) | https://doi.org/10.1080/09687680010028762
McCarty M. et al.: 2018. “Dietary Glycine Is Rate-Limiting for Glutathione Synthesis and May Have Broad Potential for Health Protection.(2018) Ochsner Journal | https://www.ochsnerjournal.org/content/ochjnl/18/1/81.full.pdf
Ning J. et al: Clinical and genetic analysis of nonketotic hyperglycinemia: A case report. (2022) World J Clin Cases | https://doi.org/10.12998/wjcc.v10.i22.7982
Razak M. et al.: Multifarious Beneficial Effect of Nonessential Amino Acid, Glycine: A Review. (2017) Oxid Med Cell Longev | https://doi.org/10.1155/2017/1716701
Ruggiero T. et al.: Treatment of symptomatic oral mucositis with sodium hyaluronate and synthetic amino acid precursors of collagen in patients undergoing haematopoietic stem cell transplantation. (2018) J Biol Regul Homeost Agents | https://pubmed.ncbi.nlm.nih.gov/29921408/
Shelkowitz E. et al.: Ketogenic diet as a glycine lowering therapy in nonketotic hyperglycinemia and impact on brain glycine levels.(2022) Orphanet J Rare Dis | https://doi.org/10.1186/s13023-022-02581-6
Wang W. et al.: Glycine metabolism in animals and humans: implications for nutrition and health. (2013) Amino Acids | https://doi.org/10.1007/s00726-013-1493-1