History & evolution
Biosynthesis vs. dietary uptake
AABA in health and disease
GABA and neurology
GABA and the gastrointestinal tract
GABA and diabetes
History and evolution
1883: GABA first synthesized (Vlainic and Jembrek 2018) | 1950: GABA found in mammalian brains | 1967: discovery of GABA’s role as inhibitory neurotransmitter
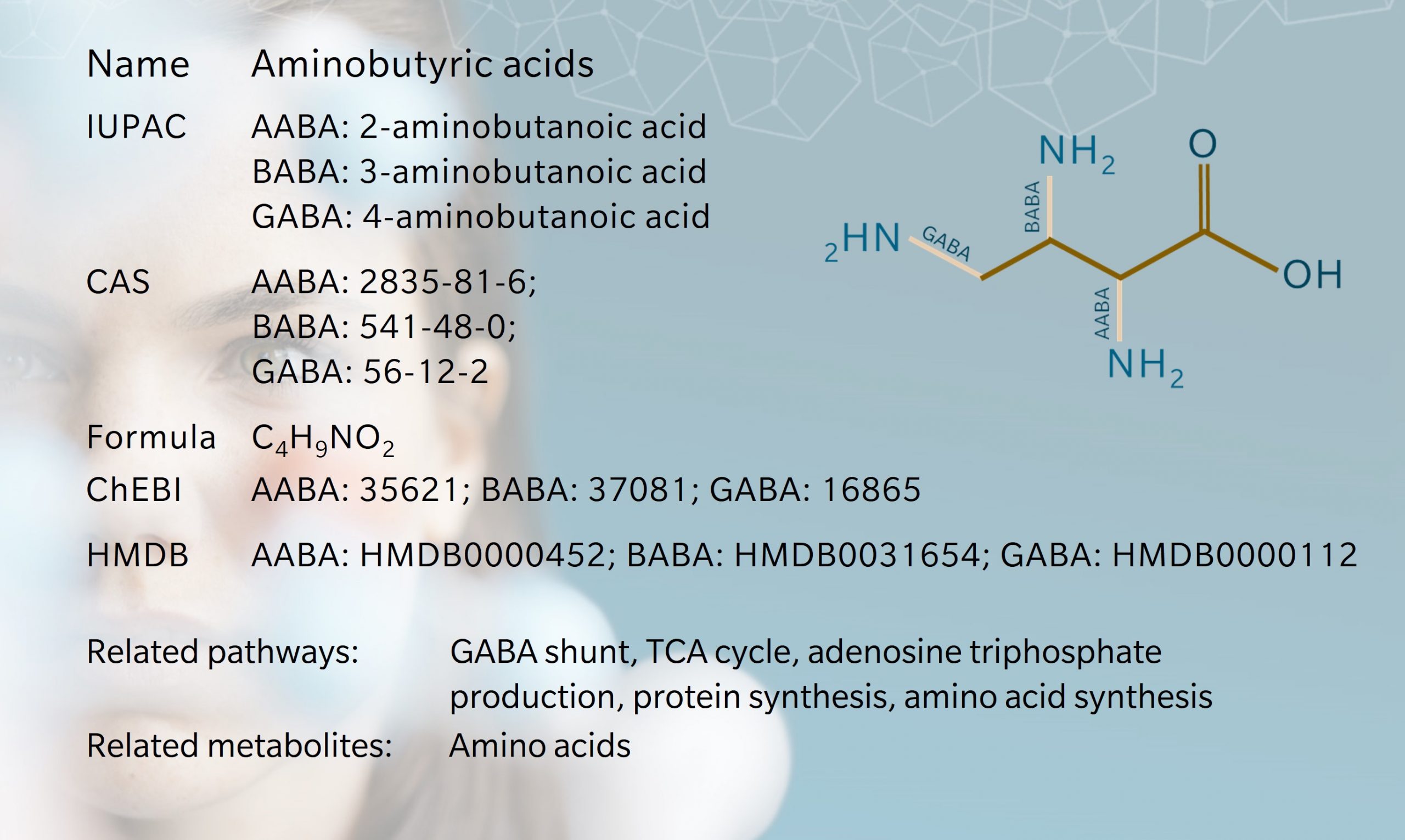
Alpha-, beta- and gamma-aminobutyric acids (AABA, BABA and GABA) are a group of structurally similar nonproteinogenic amino acids.
GABA is the most well-known and widely studied. Initially thought to exist only in plants and microbes, GABA was first discovered in rotten pancreas in 1912 (Obata et al. 2013). Its presence in human and animal brains was confirmed by Roberts and Awapara in 1950, using paper chromatography (Awapara et al. 1950, Roberts and Frankel 1950). Since then, GABA has been the subject of extensive research as the main inhibitory neurotransmitter in the central nervous system.
GABA is also involved in the regulation of many other physiological processes in tissues such as blood vessels, skeletal muscles, gastrointestinal tract, pituitary, thyroid, adrenal gland and thymus (Watanabe et al. 2002). Drugs that target the GABA system are used to treat a variety of conditions, including anxiety disorders, epilepsy, hypertension and insomnia (Evenseth et al. 2020).
AABA and BABA are isomers of GABA. AABA is found in smaller amounts in the human body, though remains a metabolite of interest for its potential effects on neurotransmission. BABA is a rare amino acid found in certain bacteria and plants, but not commonly found in humans. Interestingly, particles returned by the JAXA Hayabusa mission to asteroid Itokawa found traces of BABA, with sufficiently high levels to suggest that they were of non-terrestrial origin (Parker et al. 2021).
The term “aminobutyric acid” refers to the molecules’ structure. Each contains a butyric acid molecule with an amino group attached to one end. They are named according to the position of the amino group. Thus, in an AABA molecule, the amino group is attached to the first carbon in the butyric acid molecule (the alpha position), BABA has an amino group attached to the second carbon, and in a GABA molecule, the amino group is attached to the third carbon.
Biosynthesis vs. dietary uptake
Aminobutyric acids can be obtained from the diet or synthesized in the body. GABA is found in a variety of foods, particularly in tea, tomato, pulses and fermented foods, like kimchi and miso (Sahab et al. 2020). It’s not clear how much GABA is absorbed through the diet, though some studies suggest that consuming GABA-rich foods may increases GABA levels in the body (Hepsomali et al. 2020).
Around 30% of cerebral neurons contain GABA (Gladkevich et al. 2006). GABA is synthesized in vivo in the central nervous system by a metabolic pathway that bypasses the TCA cycle, known as the GABA shunt (Olsen et al. 1999). First, alpha-ketoglutarate generated by the TCA cycle produces glutamate. The enzyme glutamic acid decarboxylase (GAD) converts glutamate to GABA, using pyridoxal phosphate (vitamin B6) as a cofactor. GABA can be broken down by the enzyme GABA transaminase (GABA-T) to form succinic semialdehyde. Enzymes convert this to succinate, which then enters the TCA cycle, completing the “shunt”.
In the gut, it’s thought that bacteria such as Lactobacillus and Bifidobacterium use glutamate as a substrate, producing GABA through an enzymatic reaction with GAD (Pokusaeva et al. 2017). GABA may also be synthesized to a lesser extent in the pancreas, kidneys and liver.
AABA can be synthesized through various metabolic pathways, through the conversion of precursor molecules by specific enzymes. These include transamination, decarboxylation, and deamination of other amino acids, such as threonine, methionine, serine or isoleucine (HMDB 2023).
AABA in health and disease
AABA is thought to have antioxidant effects and is a biomarker for alcohol liver injury, sepsis, malnutrition, depression, and osteoporosis (Effros et al. 2011). AABA is elevated in the plasma of children with diseases such as Reye’s syndrome. (Wang et al. 2020)
Recent research using metabolomics shows the ratio of GABA and AABA is closely associated with aging-related physical performance (Wang et al. 2023). Therefore, these metabolites could be used as biomarkers to predict correlations among aging, physical performance and effects of physical activity, and potentially lead to therapeutic targets for muscle disorders such as stiff man syndrome and sarcopenia.
A study of 918 older men confirmed a relationship between branched chain amino acids and other amino acids, including AABA, with cardiovascular risk factors and mortality (LeCouteur et al. 2020). Frail participants were found to have lower levels of AABA.
GABA and neurology
Since Roberts’ discovery in 1957, GABA’s role as an inhibitory neurotransmitter has been heavily researched. GABA is the most abundant inhibitor of neurotransmission, making it an appealing target for the management of neurological disorders.
For example, epilepsy is characterized by an overexcitation of neurons, and in animal models, GABA has been shown to regulate cortical activity and predisposition to epileptic seizures (Benarroch et al. 2021). Decreased GABA levels have also been found in severe cases of Alzheimer’s disease, and dementia-associated agitation can be treated with the GABA analog, Gabapentin (Solas et al. 2015, Roane et al. 2000).
GABA may also inhibit dopamine release. Dopamine’s association with impulsive behavior therefore makes GABA a metabolite of interest in treatments for addiction and mood disorders (Trujillo et al. 2022). Similarly, GABA has also been investigated as a potential therapeutic target for Parkinson’s disease, in which dopamine is suppressed, though results are mixed. Notably, in patients with Parkinson’s disease, motor cortex GABA levels have been found to be inversely correlated with disease severity, in a study using magnetic resonance spectroscopy (Nuland et al. 2020). This suggests that GABA depletion may contribute to increased motor symptom expression.
GABA is known to reduce stress and promote sleep, but clinical trials investigating the effect of oral GABA intake for stress and sleep have had mixed results (Hepsomali et al. 2020).
Another outstanding question concerns GABA’s ability to penetrate the blood-brain barrier (BBB) (Hepsomali et al. 2020). Historically, researchers believed that GABA could not cross the BBB. However, recent research offers mixed accounts, with some researchers suggesting that small amounts of GABA can cross the BBB, and others finding larger amounts can cross. GABA’s presence in the enteric nervous system may suggest a mechanism of action on the peripheral nervous system via the gut-brain axis (Cryan et al. 2012).
GABA and the gastrointestinal tract
GABA is an established mediator of gastrointestinal function. GABAergic signaling is mediated by different types of GABA receptors, regulating both motor and secretory GI activity. Recent studies suggest that GABA may play a role in neuroimmune reactions associated with enteric inflammatory conditions, such as inflammatory bowel disease (IBD) (Auteri et al. 2015).
Treatment of IBD and irritable bowel syndrome (IBS) focus mostly on symptom relief. However, given the presence of GABA receptors in the GI system, GABAergic drugs such as benzodiazepines may offer a more comprehensive treatment (Jembrek et al. 2017). The effects of benzodiazepines include neuroimmunomodulation, pain relief and anxiolytic action.
In a study of patients with diarrhea-predominant IBS (IBS-D), patients were found to have lower levels of GABA compared to controls (Aggarwal et al. 2018). In vitro studies showed that a GABA antagonist could block the inhibitory effect of GABA, again suggesting they may be used to treat IBS-D and other inflammatory diseases.
GABA and diabetes
GABA may also be an effective treatment for type 1 diabetes. In experimental studies with rodent and human beta-cells, GABA has been shown to reverse diabetes by stimulating beta-cell regeneration (Wan et al. 2015). In addition to cell proliferation and antiapoptotic effects, it has also been found to prevent insulitis in preclinical models, suggesting that GABA may be a potential preventative treatment, too.
References
Aggarwal et al.: Dysregulation of GABAergic Signalling Contributes in the Pathogenesis of Diarrhea-predominant Irritable Bowel Syndrome. (2018) J Neurogastroenterol Motil | https://doi.org/10.5056/jnm17100
Auteri et al.: GABA and GABA receptors in the gastrointestinal tract: from motility to inflammation. (2015) Pharmacol Res | https://doi.org/10.1016/j.phrs.2014.12.001
Awapara et al.: Free γ-aminobutyric acid in brain. (1950) J. Biol. Chem | https://www.jbc.org/article/S0021-9258(19)50926-7/pdf
Benarroch et al.: What Is the Role of GABA Transporters in Seizures? (2021) Neurology | https://doi.org/10.1212/WNL.0000000000012574
Cryan et al.: Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. (2012) Neurology | https://doi.org/10.1212/10.1038/nrn3346
Effros et al.: Alpha aminobutyric acid, an alternative measure of hepatic injury in sepsis? (2011) Translational Research | https://doi.org/10.1016/j.trsl.2011.07.003
Evenseth et al.: The GABAB Receptor-Structure, Ligand Binding and Drug Development. (2020) Molecules | https://doi.org/10.3390/molecules25133093
Gladkevich et al.: The peripheral GABAergic system as a target in endocrine disorders. (2006) Auton Neurosci | https://doi.org/10.3390/molecules25133093
Hepsomali et al.: Effects of Oral Gamma-Aminobutyric Acid (GABA) Administration on Stress and Sleep in Humans: A Systematic Review. (2020) Front Neurosci | https://doi.org/10.3389/fnins.2020.00923
Jembrek et al.: GABAergic System in Action: Connection to Gastrointestinal Stress-related Disorders. (2017) Curr Pharm Des | https://doi.org/10.2174/1381612823666170209155753
LeCouteur et al.: Branched Chain Amino Acids, Cardiometabolic Risk Factors and Outcomes in Older Men: The Concord Health and Ageing in Men Project. (2020) The Journals of Gerontology | https://doi.org/10.1093/gerona/glz192
Obata et al.: Synaptic inhibition and γ-aminobutyric acid in the mammalian central nervous system. (2013) Proc Jpn Acad Ser B Phys Biol Sci | https://doi.org/10.2183/pjab.89.139/
Olsen et al.: GABA Synthesis, Uptake and Release. (1999) In Basic Neurochemistry: Molecular, Cellular and Medical Aspects. 6th edition. | https://www.ncbi.nlm.nih.gov/books/NBK27979/
Parker et al.: Extraterrestrial Non-Protein Amino Acids Identified In Carbon-Rich Particles Returned From Asteroid Itokawa. (2021) 84th Annual Meeting of The Meteoritical Society 2021 | https://doi.org/10.1111/maps.13794
Pokusaeva et al.: GABA-producing Bifidobacterium dentium modulates visceral sensitivity in the intestine. (2017) Neurogastroenterology & Motility | https://doi.org/10.1111/nmo.12904
Roane et al.: Treatment of Dementia-Associated Agitation With Gabapentin. (2000) Neuropsychiatry Clin Neurosci | https://doi.org/10.1176/jnp.12.1.40
Roberts and Frankel: γ-Aminobutyric acid in brain: its formation from glutamic acid. (1950) J. Biol. Chem | https://www.jbc.org/article/S0021-9258(19)50929-2/pdf
Sahab et al.: γ-Aminobutyric acid found in fermented foods and beverages: current trends. (2020) Neuropsychiatry Clin Neurosci | https://doi.org/10.1016/j.heliyon.2020.e05526
Solas et al.: Treatment Options in Alzheimer`s Disease: The GABA Story. (2015) Curr Pharm Des | https://doi.org/10.1016/j.heliyon.2020.e05526
Trujillo et al.: Dopamine-induced changes to thalamic GABA concentration in impulsive Parkinson disease patients. (2022) npj Parkinson’s Disease | https://doi.org/10.1038/s41531-022-00298-8
van Nuland et al.: GABAergic changes in the thalamocortical circuit in Parkinson’s disease. (2020) npj Parkinson’s Disease | https://doi.org/10.1002/hbm.24857
Vlainic et al.: GABA (γ-Aminobutyric Acid). (2018) In Encyclopedia of Signaling Molecules by S. (eds) Choi. Springer, Cham.
Wan et al.: GABAergic system in the endocrine pancreas: a new target for diabetes treatment. (2015) Diabetes Metab Syndr Obes | https://doi.org/10.2147/DMSO.S50642
Wang et al.: γ-Aminobutyric acids (GABA) and serum GABA/AABA (G/A) ratio as potential biomarkers of physical performance and aging (preprint). (2023) Research Square | https://doi.org/10.21203/rs.3.rs-2492780/v1
Wang et al.: Quantification of aminobutyric acids and their clinical applications as biomarkers for osteoporosis. (2020) Commun Biol | https://doi.org/10.1038/s42003-020-0766-y
Watanabe et al.: GABA and GABA receptors in the central nervous system and other organs. (2002) Int Rev Cytol | https://doi.org/10.1016/s0074-7696(02)13011-7