History & Evolution
1895: discovery (Haiser 1895) | 1908: first synthesis (Jacobs et al. 1908) | 1970: crystal structure discovered (Munns et al. 1970)
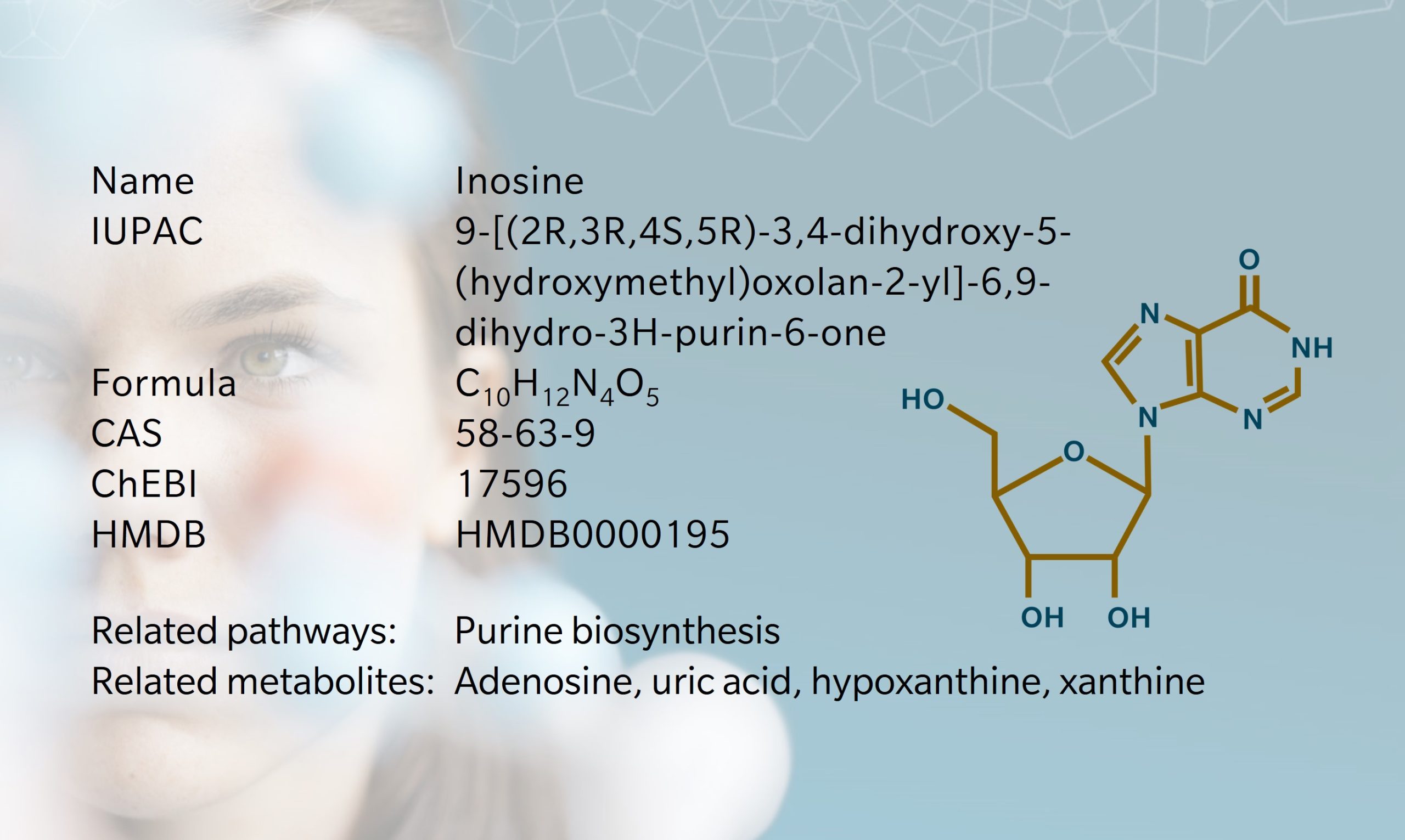
Inosine, a nucleoside present in all living cells, has an important role in purine biosynthesis, gene translation and modulation of the fate of RNAs (Srinivasan et al. 2021). Consisting of ribose and hypoxanthine, inosine is structurally similar to adenosine, differing only by the presence of a keto group at position 6 of the purine ring instead of an amino group. The exact date of discovery is somewhat ambiguous, however papers discussing inosine and inosinic acid date back to the 19th century (Haiser 1895). The first synthesis of inosine was achieved by treating adenosine with sodium nitrate and acetic acid (Jacobs et al. 1908). When the first tRNA structure was published, inosine was one of the first nucleobase modifications discovered (Holley et al. 1965). Soon after, its molecular and crystal structures were identified (Munns et al. 1970). Inosine is involved in the regulation of multiple mechanisms, including RNA editing, metabolic enzyme activity, and several signaling pathways. Recent studies demonstrated its anti-inflammatory, immunomodulatory, and neuroprotective effects (Wang et al. 2023; Kim et al. 2022; Basile et al. 2022).. Inosine has a critical role as a regulator of immune checkpoint inhibition, thereby modulating the response to therapy in various cancer types (Jeyaraj et al. 2024).
Biosynthesis vs. dietary uptake
Inosine can be obtained through dietary intake as well as endogenous biosynthesis. Red meat, pork, fish, and poultry products are rich inosine sources (Kaneko et al. 2020), and its 5’-monophosphate is associated with the flavor and taste of red and white meats. Inosine biosynthesis occurs both intracellularly and extracellularly. Endogenous inosine can be synthesized by three different routes (Kim et al. 2022):
• Adenosine deaminases (ADA) catalyze the irreversible hydrolytic deamination of adenosine, substituting the amino group by a keto group. The deamination of adenosine to inosine occurs predominantly when intracellular concentrations of adenosine are high, which is associated with cellular stress conditions like hypoxia and ischemia (Haskó et al. 2004).
• 5′-nucleotidase (5’NT) reversibly dephosphorylates inosine monophosphate (IMP) and other 5’-nucleotides, both inside and outside of cells.
• Purine nucleoside phosphorylase (PNP) can catalyze the reaction of hypoxanthine with ribose-1-phosphate (R1P) to produce inosine. However, the opposite reaction, degrading inosine, is often favored due to high levels of inorganic phosphate than R1P.
Besides endogenous biosynthesis, gut microbiota can produce inosine which is absorbed by the host to enter the circulation. Another source of inosine is specific supplementation in the form of isoprinosine as immunostimulant.
In the human body, inosine can be metabolized to xanthine, and further to hypoxanthine and uric acid, which is one of the major antioxidants and protects against neurological and intestinal diseases (Cipriani et al. 2010).
Inosine and the microbiome

There is a strong relationship between inosine and microbiota. It is produced by several species of the gut microbiome that are considered beneficial for the host, including Bifidobacterium pseudolongum and Akkermansia muciniphila, and modulates host immune and inflammatory functions (Brown et al. 2021; Mager et al. 2020). Gut microbiota remodeling by enrichment of B. pseudolongum significantly increased anticancer immunotherapy response (Mager et al. 2020). In mouse models, remodeling microbiota with Lactobacillus reuteri prolonged survival, reduced multiorgan inflammation, and restored levels of inosine. Feeding inosine directly extended the rodent life span and inhibited multiorgan inflammation (He et al. 2017). Administration of Bifidobacterium infantis exhibited cardioprotective effects via its metabolite inosine by suppressing proinflammatory cytokine production and cardiac inflammation (Zhang et al. 2024). In pediatric obesity patients, Eubacterium hallii, Ruminococcus gnavus, and Dorea as an obesogenic genus were found to be significantly and positively correlated with serum inosine and uric acid (Lee et al. 2023).
Inosine and cancer
Inosine’s role in cancer is complex and context-dependent. It associates with both tumor progression and anti-cancer immune response. Indeed, inosine is part of the adenosine pathway, which is essential to maintain a balanced immune homeostasis. Within this pathway, the energy metabolism-related forms adenosine triphosphate and diphosphate (ATP and ADP) may act in a pro-inflammatory manner, whereas circular AMP (cAMP) is considered an anti-inflammatory signaling molecule (Mager et al. 2020). Inosine, as a product of adenosine metabolism, is a promising biomarker linked to inflammation, cancer, tumor progression, metastasis, and drug resistance (Jeyaraj et al. 2024). Changes in inosine levels have been shown to associate with several cancer types, although with inconsistent findings. Inosine levels were increased in patients with lung squamous cell carcinoma, esophageal squamous cell carcinoma, head and neck squamous cell carcinoma, and bladder cancer. However, its levels were decreased in pancreatic cancer patients (Kim et al. 2022; Cheng et al. 2024). The role of inosine in immune checkpoint inhibition (ICI) therapy was shown in several studies recently where it aids in anticancer treatment by giving energy to T cells in a glucose-restricted environment, such as the tumor microenvironment (Samami et al. 2023; Klysz et al. 2023). It has been reported that it causes T cell-mediated tumor-killing activity and acts through the inhibition of inflammatory cytokines (Mager et al. 2020).
These data collectively suggest that inosine functions as a potential biomarker for prediction of cancer risk, drug response, and early detection of metastasis of various tumors. Regarding cancer treatment, there is a clear need for more clinical studies to better understand its roles and effects (Kim et al. 2022).
Inosine and neurology
Adenosine and its metabolite inosine can permeate the blood-brain barrier. Therefore, a change in plasma or serum level may reflect a change in central nervous system (CNS) and systemic inosine administration can affect the CNS (Nascimento et al. 2021) . Increased levels of inosine have been measured in the cerebrospinal fluid of acute and chronic pain patients (Schmidt et al. 2010) and in the serum of fibromyalgia patients (Fais et al. 2013). In both children and adolescents suffering from major depressive disorder (MDD), decreased levels of inosine in plasma were identified as a diagnostic biomarker with high discriminative performance and correlated with the severity of depression symptoms. Furthermore, inosine concentrations increased after treatment with antidepressants, suggesting that inosine is indicative of an antidepressant effect (Zhou et al. 2019). However, inosine levels were also decreased in patients with recurrent MDD in remission, suggesting a complex regulation of inosine levels in MDD (Mocking et al. 2021).
Patients with Parkinson’s disease and Alzheimer’s disease display decreased uric acid levels in the serum according to several studies (Cipriani et al. 2010). In a placebo-controlled clinical study, serum and cerebrospinal fluid uric acid levels could be increased by inosine administration, which resulted in delayed PD progression, particularly in women (Schwarzschild et al. 2014; Schwarzschild et al. 2019). In another clinical trial, inosine was co-administered with febuxostat, a xanthine oxidase inhibitor used to treat gout and hyperuricemia, providing an improvement in Parkinson’s symptoms (Watanabe et al. 2020). In mice models of neuronal injury, inosine administration resulted in axon regeneration and motor improvement (Soares dos Santos Cardoso, Fellipe et al. 2019). Furthermore, inosine may contribute to neural plasticity and axonal sprouting by affecting gene expression in the CNS (Nascimento et al. 2021). These properties could also explain how inosine treatment prevented memory deficits and enhanced anti-inflammatory cytokine levels in the brain in a rat model of Alzheimer’s disease (Teixeira et al. 2022).
Inosine and dietary/microbiome intervention
Oral application of inosine-based drugs may be effective against viral infections, neurological and autoimmune diseases. Isoprinosine, a synthetic complex of inosine and dimethylaminoisopropanol, is a very effective treatment agent to enhance the natural immune response of lymphocytes after various viral infections including influenza (Pavlova et al. 2018) and COVID-19 (Beran et al. 2020). In primate models, human immunodeficiency virus type 1/simian immunodeficiency virus (HIV-1/SIV) infection was related to elevated inosine levels as well as immune activation and disease progression markers (He et al. 2015).
In conclusion, inosine is a critical metabolite in human metabolism with diverse roles in health and disease. Inosine can cross the blood-brain barrier, showing promise as a biomarker and potential therapeutic agent for neurological diseases like MDD, Parkinson’s disease, and Alzheimer’s disease. Its role in immune homeostasis and inflammation makes inosine an important player in infectious diseases and metabolism of various cancers. Use of the approved inosine-containing medication, inosine pranobex, and clinical trials in Parkinson’s disease give promising results and underlines its potential in clinical applications.
References
Basile, M. et al.: Inosine in Neurodegenerative Diseases: From the Bench to the Bedside (2022) Molecules | DOI: 10.3390/molecules27144644.
Beran, J. et al.: Inosine Pranobex Significantly Decreased the Case-Fatality Rate among PCR Positive Elderly with SARS-CoV-2 at Three Nursing Homes in the Czech Republic (2020) Pathogens (Basel, Switzerland) | DOI: 10.3390/pathogens9121055.
Brown, E.M. et al.: Gut microbiome ADP-ribosyltransferases are widespread phage-encoded fitness factors (2021) Cell host & microbe | DOI: 10.1016/j.chom.2021.07.011.
Cheng H. et al.: Adenosine-to-Inosine RNA editing in cancer: molecular mechanisms and downstream targets (2024) Protein & Cell | DOI: 10.1093/procel/pwae039
Cipriani, S. et al.: Urate: a novel biomarker of Parkinson’s disease risk, diagnosis and prognosis (2010) Biomarkers in Medicine | DOI: 10.2217/bmm.10.94.
Fais, A. et al.: Purine metabolites in fibromyalgia syndrome (2013) Clinical biochemistry | DOI: 10.1016/j.clinbiochem.2012.09.009.
Haiser, F.: Zur Kenntniss der Inosinsäure (1895) | DOI: 10.1007/BF01519001
Haskó, G. et al.: Immunomodulatory and neuroprotective effects of inosine (2004) Trends in Pharmacological Sciences | DOI: 10.1016/j.tips.2004.01.006.
He, B. et al.: Resetting microbiota by Lactobacillus reuteri inhibits T reg deficiency-induced autoimmunity via adenosine A2A receptors (2017) The Journal of experimental medicine | DOI: 10.1084/jem.20160961.
He, T. et al.: Critical Role for the Adenosine Pathway in Controlling Simian Immunodeficiency Virus-Related Immune Activation and Inflammation in Gut Mucosal Tissues (2015) Journal of virology | DOI: 10.1128/JVI.01196-15.
Holley, R.W. et al.: Structure of a ribonucleic acid (1965) Science (New York, N.Y.) | DOI: 10.1126/science.147.3664.1462.
Jacobs, W.A. et al.: Further studies on the constitution of inosinic acid (1908) Experimental Biology and Medicine | DOI: 10.3181/00379727-6-24.
Jeyaraj, F.T. et al.: Multifaceted role of inosine in complex diseases and human health (2024) Nutrition reviews | DOI: 10.1093/nutrit/nuae029
Kaneko, K. et al.: Determination of total purine and purine base content of 80 food products to aid nutritional therapy for gout and hyperuricemia (2020) Nucleosides, Nucleotides & Nucleic Acids | DOI: 10.1080/15257770.2020.1748197.
Kim, I.S. et al.: Inosine: A bioactive metabolite with multimodal actions in human diseases (2022) Frontiers in pharmacology | DOI: 10.3389/fphar.2022.1043970.
Klysz, D. et al.: Inosine endows CAR T cells with features of increased stemness and anti-tumor potency (2023) Cancer Res | DOI: 10.1158/1538-7445.AM2023-1158
Lee, Y. et al.: Serum, Urine, and Fecal Metabolome Alterations in the Gut Microbiota in Response to Lifestyle Interventions in Pediatric Obesity: A Non-Randomized Clinical Trial (2023) Nutrients | DOI: 10.3390/nu15092184.
Mager, L.F. et al.: Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy (2020) Science (New York, N.Y.) | DOI: 10.1126/science.abc3421.
Mocking, R.J.T. et al.: Metabolic features of recurrent major depressive disorder in remission, and the risk of future recurrence (2021) Translational Psychiatry | DOI: 10.1038/s41398-020-01182-w.
Munns, A.R. et al.: The crystal and molecular structure of inosine (1970) Acta crystallographica. Section B: Structural crystallography and crystal chemistry | DOI: 10.1107/s0567740870003679.
Nascimento, F.P. et al.: Inosine as a Tool to Understand and Treat Central Nervous System Disorders: A Neglected Actor? (2021) Frontiers in neuroscience | DOI: 10.3389/fnins.2021.703783.
Pavlova, E.L. et al.: Combined efficacy of oseltamivir, isoprinosine and ellagic acid in influenza A(H3N2)-infected mice (2018) Biomedicine & pharmacotherapy | DOI: 10.1016/j.biopha.2017.12.014.
Samami, E. et al.: Inosine, gut microbiota, and cancer immunometabolism (2023) American journal of physiology. Endocrinology and metabolism | DOI: 10.1152/ajpendo.00207.2022.
Schmidt, A.P. et al.: Changes in purines concentration in the cerebrospinal fluid of patients experiencing pain: a case-control study (2010) Neuroscience Letters | DOI: 10.1016/j.neulet.2010.02.067.
Schwarzschild, M.A. et al.: Inosine to increase serum and cerebrospinal fluid urate in Parkinson disease: a randomized clinical trial (2014) JAMA neurology | DOI: 10.1001/jamaneurol.2013.5528.
Schwarzschild, M.A. et al.: Sex differences by design and outcome in the Safety of Urate Elevation in PD (SURE-PD) trial (2019) Neurology | DOI: 10.1212/wnl.0000000000008194.
Soares dos Santos Cardoso, Fellipe et al.: Inosine Accelerates the Regeneration and Anticipates the Functional Recovery after Sciatic Nerve Crush Injury in Mice (2019) Neuroscience | DOI: 10.1016/j.neuroscience.2019.09.023.
Srinivasan, S. et al.: Inosine in Biology and Disease (2021) Genes | DOI: 10.3390/genes12040600.
Teixeira, F.C. et al.: Investigating the Effect of Inosine on Brain Purinergic Receptors and Neurotrophic and Neuroinflammatory Parameters in an Experimental Model of Alzheimer’s Disease (2022) Molecular neurobiology | DOI: 10.1007/s12035-021-02627-z.
Wang, N. et al.: A broad-spectrum anti-inflammatory against SARS-CoV-2 infection-induced acute lung injury via suppressing TBK1 phosphorylation (2023) | Journal of Pharmaceutical Analysis| DOI: 10.1016/j.jpha.2022.10.002.
Watanabe, H. et al.: Improved Parkinsons disease motor score in a single-arm open-label trial of febuxostat and inosine (2020) Medicine | DOI: 10.1097/MD.0000000000021576.
Zhang, H. et al.: Prophylactic supplementation with Bifidobacterium infantis or its metabolite inosine attenuates cardiac ischemia/reperfusion injury (2024) iMeta | DOI: 10.1002/imt2.220.
Zhou, X. et al.: Polyunsaturated fatty acids metabolism, purine metabolism and inosine as potential independent diagnostic biomarkers for major depressive disorder in children and adolescents (2019) Molecular psychiatry | DOI: 10.1038/s41380-018-0047-z.