- History & Evolution
- Structure and nomenclature
- Biosynthesis vs. dietary uptake
- Triglyceride storage and mobilization
- Triglycerides and fatty liver disease
- Triglycerides, insulin and diabetes
- Triglycerides and cardiovascular diseases (CVD)
History & Evolution
1929: first analysis of lipoprotein composition | 1956: first enzymatic synthesis of triglycerides (Weiss and Kennedy 1956) | 1960s: first understanding of fat transport involving triglycerides (Olson 1998)
Triglycerides are complex molecules used for the storage and transport of non-soluble fatty acids in the body. They help transport fat and glucose from the liver, circulating continuously throughout the vascular system. When metabolized, they are a major source of energy in mitochondria. Triglycerides are sourced from consumption of high-fat foods, or synthesized in the liver or adipose tissue (Alves-Bezerra and Cohen 2017).
The idea that fat may be transported around the body via the blood first emerged as early as the mid 17th century. Over the next three hundred years, our understanding of the fat transport system evolved as analytical techniques improved (Litchfield 1972, Olson 1998).
While triglycerides play an essential role in human health, elevated levels are associated with health problems such as obesity, cardiovascular disease, non-alcoholic fatty liver disease (NAFLD) and pancreatitis. Lifestyle factors are most often to blame, particularly a high-fat diet, but high triglycerides levels can also be attributed to a genetic condition known as hypertriglyceridemia. Some medical conditions such as kidney disease, type 2 diabetes, and thyroid disorders can also affect plasma triglyceride levels (Pejic and Ledd 2006).
Structure and nomenclature
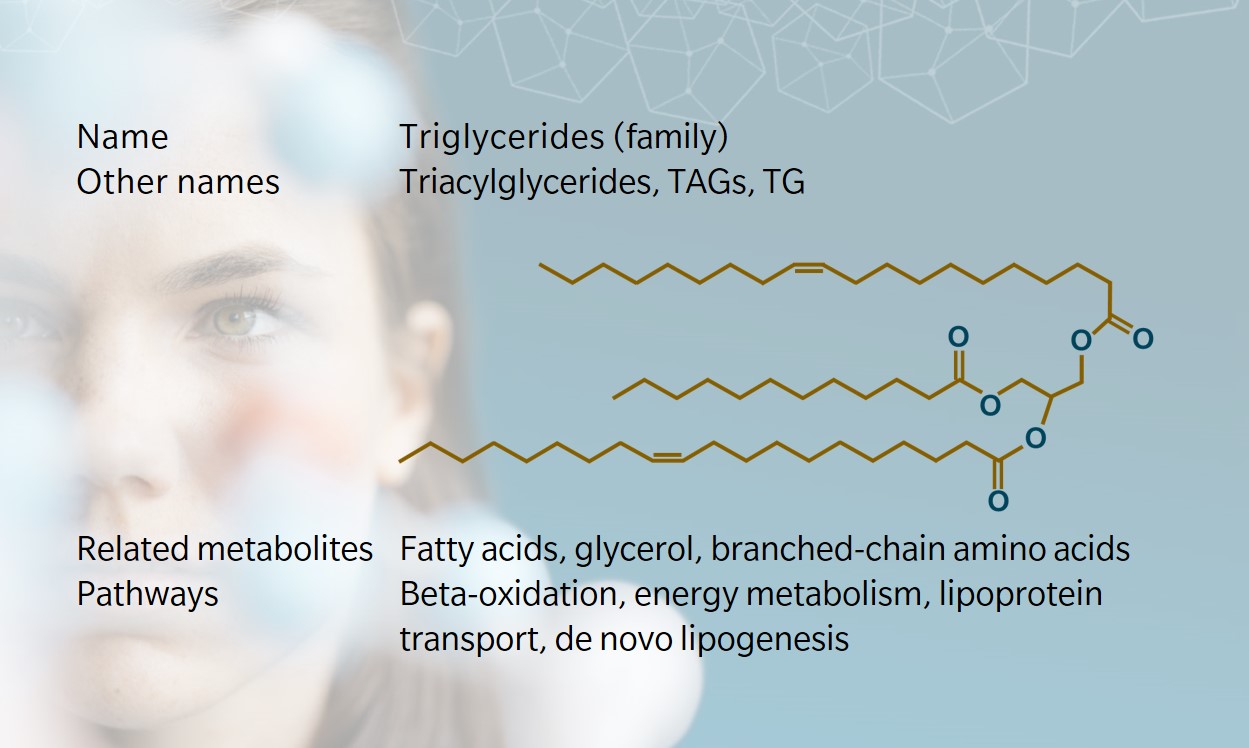
Like all lipids, triglycerides (or triacylglycerols) are composed primarily of carbon, hydrogen and oxygen atoms. They have a glycerol backbone bound to the esters of three fatty acids, and form following a condensation reaction. Fatty acids have chains of varying numbers of carbon atoms, which are joined either by single bonds (saturated fats), double bonds (unsaturated fats), or multiple double bonds (polyunsaturated fats). The tightly formed carbon atoms in saturated fats means these are usually solid at room temperature, like butter. Unsaturated fats are looser and are more likely to be liquids. Most naturally occurring fats contain a range of triglycerides, which means they melt at varying temperatures.
Triglycerides may contain three identical or three different fatty acids. Triglycerides with no double carbon bonds are structured in a straight zigzag. Those with double bonds have either a cis or trans configuration, depending on whether the hydrogen atoms are on the same side (cis) or opposite sides (trans) (National Research Council (US) Committee on Diet and Health 1989).
Simple triglycerides, where all three fatty acid esters are the same, take their common name from the fatty acid from which they are derived, such as palmitin, derived from palmitic acid. Scientific nomenclature varies, describing the number of carbons and double bonds in the whole molecule with varying degrees of detail. At biocrates, we use a nomenclature where one fatty acid is counted individually and the other two are summed. For example, TG(20:1_32:1) has one fatty acid with 20 carbons and one double bond, plus two fatty acids that add up to 32 carbons and one double bond. Thus, several TGs fit this description, such as the one depicted on the metabolite card above.
Biosynthesis vs. dietary uptake
Triglycerides contribute around 90-95% of dietary fat (Iqbal and Hussain 2009), and are found in many food sources, from dairy products to meat. Bile salts in the liver break the fat into micelles, which are hydrolyzed by pancreatic lipase to form fatty acids and monoglycerides (Bayly 2014). Through de novo lipogenesis (DNL), these are then turned back into triglycerides in enterocytes, combining cholesterol and proteins to form chylomicrons, which are triglyceride-rich lipoproteins. The chylomicrons enter the lymphatic system before being transported around the body via the blood (Alves-Bezerra and Cohen 2017).
Endogenous synthesis occurs mostly in the liver and adipose tissue, but also in the intestines, muscles, brain and other organs. There are several pathways to triglycerides synthesis, including the glycerol-3-phosphate pathway, the dihydroxyacetone phosphate (DHAP) pathway, and the monoacylglycerol pathway (Tracey et al. 2018). Each involves the enzymatic conversion of fatty acids into acyl-CoA molecules. Through these processes, triglyceride molecules repeatedly assemble and disassemble to cross membrane barriers and make sure energy gets to the right organs.
Other metabolites such as glucose, fructose, acetate and the branched-chain amino acids leucine and isoleucine also serve as precursors of acetyl-CoA and contribute carbons to the de novo synthesis of fatty acids that will enter DNL (Wallace 2020).
Triglyceride storage and mobilization
The liver metabolizes large amounts of fatty acids, but stores less than 5% as triglycerides (Alves-Bezerra and Cohen 2017). Most is released in very low density lipoproteins (VLDLs) and then converted to low density lipoproteins (LDLs) in plasma, before being stored elsewhere in the body (Spector 1984, Olson 1998). High density lipoproteins (HDLs) absorb cholesterol, before similarly converting to LDLs for transportation around the body (Olson 1998).
Once triglycerides have been transported to the organs via plasma, they can be hydrolyzed again to cross membranes. In adipose tissue, triglycerides re-form for storage in fat droplets. Each cell in adipose tissue contains a fat droplet, and when energy is required, glucagon and adrenaline activate hormone-sensitive lipase (HSL) to mobilize the triglycerides in the cell.
Triglycerides and fatty liver disease
Because the liver is the main site of fatty acid metabolism, it’s the first responder to a high triglyceride diet. A diet that’s too high in dietary fats can affect hepatic fatty acid metabolism, causing a build-up of triglycerides within the hepatocytes – a fatty liver. Ultimately, this can lead to non-alcoholic fatty liver disease (NAFLD) (Alves-Bezerra and Cohen 2017).
Fatty liver used to be considered a sign of alcoholism, until it became clear that people (including children) could suffer from a fatty liver without drinking excessively. Alcohol does cause a fatty liver, but so does a diet high in sugar and fats, especially when combined with a sedentary lifestyle.
High levels of triglycerides are a key marker of NAFLD, alongside liver damage and inflammation (Donnelly et al. 2005). Pathogenesis of NAFLD isn’t completely understood, though recent studies suggest a “multiple-hit” of complex factors contributing to the disease (Nobili et al. 2013, Svegliati-Baroni et al. 2019).
Lipotoxicity, gut dysbiosis and metabolic dysfunction appear to activate free radicals in the liver. Insulin resistance can trigger triglyceride synthesis and hepatic lipid storage. However, new studies suggest triglycerides may have a potential protective effect against NAFLD progression in mice (Yamaguchi et al. 2007).
Triglycerides, insulin and diabetes
As noted, most triglycerides are stored in adipose tissue. Human and animal models show links between lower rates of DNL in adipose tissue and a higher likelihood of insulin resistance (Wallace 2020). Insulin signaling triggers the mobilization of triglycerides stores (Sears and Perry 2015). It keeps triglycerides levels in check by enabling the synthesis of lipase, the enzyme needed for triglyceride hydrolysis (Sadur and Eckel 1982).
Omics-based techniques are proving useful in furthering understanding of the metabolites and lipids involved in diabetes and related conditions. For example, a systematic review of studies using metabolomic techniques to investigate pre-diabetes and type 2 diabetes confirmed the association between high concentrations of triglycerides and an elevated risk of disease (Guasch-Ferré et al. 2016).
Triglycerides and cardiovascular diseases (CVD)
While the link between non-fasting triglycerides levels and cardiovascular risk is unclear, several studies have shown a correlation between the two. By promoting insulin resistance, hypertriglyceridemia (the condition of elevated triglycerides levels) and high levels of free fatty acids contribute to several cardiovascular risks (Rygiel 2018). This is likely due to the fact that high triglycerides levels bring high LDL levels, which have atherogenic properties, increasing the risk of heart attacks, heart disease and stroke.
One study showed that elevated non-fasting triglycerides levels were indicative of an increased risk of myocardial infarction, ischemic heart disease, diabetes and other cardio-metabolic conditions (Nordestgaard et al. 2007).
However, there remains some debate about whether triglycerides are a risk factor or biomarker of cardiovascular disease, with many studies suggesting triglycerides are an important biomarker of CVD risk, but not directly atherogenic (Miller 2011). One large-scale mass spectrometry-based metabolomics study suggests monoglycerides may be a better marker than triglycerides (Ganna 2014).
Learn more about the roles of triglycerides in complex chronic diseases such as cancer, Alzheimer’s disease, depression, inflammatory bowel disease, multiple sclerosis and diabetes in our whitepaper “Complex chronic diseases have a common origin”.
References
Alves-Bezerra, M., and D. Cohen: Triglyceride Metabolism in the Liver. (2017) Comprehensive Physiology | http://doi.org/10.1002/cphy.c170012
Bayly, G.: Lipids and Disorders of Lipoprotein Metabolism.(2014) Clinical Biochemistry | https://doi.org/10.1016/B978-0-7020-5140-1.00037-7
Donnelly, K. et al.: Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease.(2005) The Journal of Clinical Investigation | http://doi.org/10.1172/JCI23621
Ganna, A. et al.: Large-scale Metabolomic Profiling Identifies Novel Biomarkers for Incident Coronary Heart Disease. (2014) PLOS Genetics | https://doi.org/10.1371/journal.pgen.1004801
Guasch-Ferré, M. et al.: Metabolomics in Prediabetes and Diabetes: A Systematic Review and Meta-analysis.(2016) Diabetes Care | https://doi.org/10.2337/dc15-2251
Iqbal, J., and M. Hussain: Intestinal Lipid Absorption. (2009) Am J Physiol Endocrinol Metab. | http://doi.org/10.1152/ajpendo.90899.2008
Litchfield , C.: Analysis of Triglycerides. (1972) New York and London: Academic Press | https://doi.org/10.1093/chromsci/11.8.7A
Miller, M. et al.: Triglycerides and Cardiovascular Disease: A Scientific Statement From the American Heart Association.” (2011) Circulation. | https://doi.org/10.1161/CIR.0b013e3182160726
National Research Council (US) Committee on Diet and Health: Diet and Health: Implications for Reducing Chronic Disease Risk (1989) National Academies Press | http://doi.org/10.17226/1222
Nobili, V. et al.: A 360-degree overview of paediatric NAFLD: Recent insights. (2013) Journal of Hepatology | https://doi.org/10.1016/j.jhep.2012.12.003
Nordestgaard, B. et al.: Nonfasting Triglycerides and Risk of Myocardial Infarction, Ischemic Heart Disease, and Death in Men and Women. (2007) JAMA | https://doi.org/10.1001/jama.298.3.299
Olson, R.: Discovery of the Lipoproteins, Their Role in Fat Transport and Their Significance as Risk Factors. (1998) The Journal of Nutrition | https://doi.org/10.1093/jn/128.2.439S
Pejic, R., and D. Ledd: Hypertriglyceridemia (2006)The Journal of the American Board of Family Medicine | https://doi.org/10.3122/jabfm.19.3.310
Rygiel, K.: Hypertriglyceridemia – Common Causes, Prevention and treatment Strategies. (2018) Current Cardiology Reviews | https://doi.org/10.2174/1573403X14666180123165542
Sadur, C., and R. Eckel: Insulin stimulation of adipose tissue lipoprotein lipase. Use of the euglycemic clamp technique.” (1982) J Clin Invest. | https://doi.org/10.1172/JCI110547
Sears, B., and M. Perry: The role of fatty acids in insulin resistance. (2015) Lipids in health and disease | https://doi.org/doi:10.1186/s12944-015-0123-1
Spector, A.: Plasma Lipid Transport. (1984) Clin Physiol Biochem | https://pubmed.ncbi.nlm.nih.gov/6386279/
Svegliati-Baroni, G. et al.: Lipidomic biomarkers and mechanisms of lipotoxicity in non-alcoholic fatty liver disease. (2019) Free Radical Biology and Medicine | https://doi.org/10.1016/j.freeradbiomed.2019.05.029
Tracey, T. et al.: Neuronal Lipid Metabolism: Multiple Pathways Driving Functional Outcomes in Health and Disease. (2018) Front Mol Neurosci. | https://doi.org/10.3389/fnmol.2018.00010
Wallace, M. et al.: Tracing insights into de novo lipogenesis in liver and adipose tissues. (2020) Seminars in Cell & Developmental Biology | https://doi.org/10.1016/j.semcdb.2020.02.012
Weiss, S., and E. Kennedy: The Enzymatic Synthesis of Triglycerides. (1956) Journal of the American Chemical Society | https://doi.org/10.1021/ja01595a088
Yamaguchi, K. et al.: Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. (2007) Hepatology | https://doi.org/10.1002/hep.21655