This is the first in a series of five blogs where I’ll discuss how metabolomics is set to transform medicine as we know it. The applications of omics in biomedical research are vast, and to help organize the different ways metabolomics can be used, I’ll discuss this in the context of 5P medicine.
An introduction to 5P medicine
5P medicine is a concept developed to address the limitations of traditional Western medicine, which typically focuses on reacting to illness or injury. Making use of five components – preventive, predictive, precision, participatory and population-based medicine – 5P medicine aims to shift the focus towards a more proactive and patient-centric practice.
Personally, I first encountered this concept in a book by Leroy Hood and Nathan Price, The Age of Scientific Wellness. Hood and Price describe what they call P4 medicine. Rather than the familiar model that treats or manages disease after its occurrence, the authors offer an alternative that leverages the scientific tools at our disposal to understand health and disease. The result is a transition from what they call a “sickcare” system towards a genuine “healthcare” system. The 5P model expands this concept by adding population-based medicine to the original four, and incorporating strategies that use the power of large cohort studies to find additional insights.
Omics and the future of research and health
Omics research has been around for over 30 years, and while genomics is gaining traction and beginning to be used in the clinics, other omics, and multiomics integration, are still lagging. Each omic addresses a distinct layer of biology, with its own codes, regulatory signals and sensitivity to external influences that offer unique insights.
Genomics is the layer least influenced by environment once a person is born. Of course, mutations can occur and change someone’s DNA, but these happen locally and are most often corrected. In contrast, metabolomics is the layer most sensitive to the environment. It responds to our diet, lifestyle, and exposures. For example, metabolomics profiles can shift in response to year after year of terrible food choices. Poor diet usually leads to chronic low-grade inflammation, which presents metabolic patterns closely associated with markers of inflammation at the granular level (Pietzner et al. 2017).
Genomics can tell you about your risk of developing an inflammatory disease, but it cannot track changes throughout your life. Metabolomics can. That’s exactly why it’s such a great tool for preventive medicine. Many chronic diseases have underlying inflammation, and by detecting changes in this “breeding ground” before the onset of disease, metabolomics offers a chance for early intervention. And for illnesses that can decrease in severity or disappear altogether, metabolomics is a unique omic tool to monitor patient status.
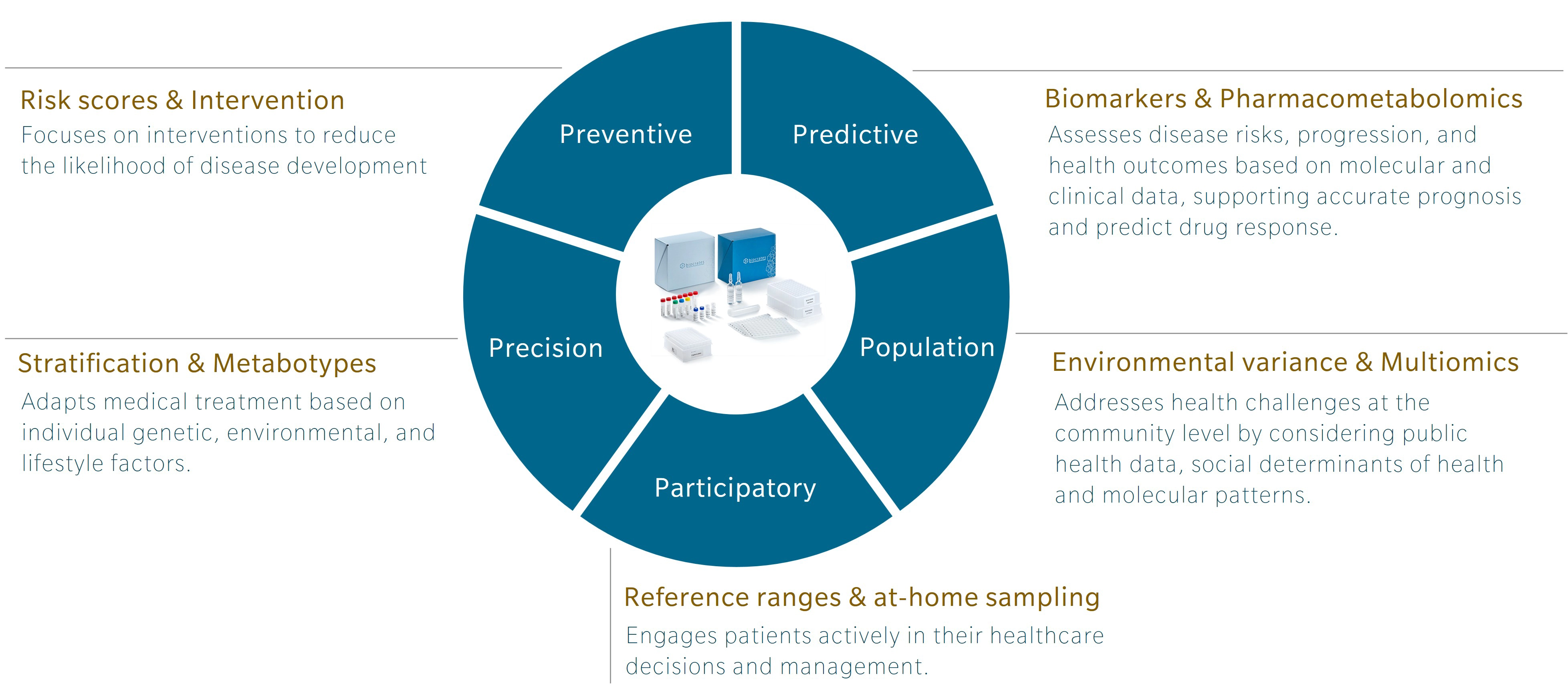
Preventive medicine with metabolomics
At the intersection of disease prevention and metabolomics, some of the most interesting applications include:
- changing what we eat (dietary intervention),
- changing our microbiome (probiotics),
- artificially increasing the levels of microbiome products (postbiotic supplements)
- identifying individuals at risk of developing disease using metabolomics.
Dietary intervention
With growing awareness of the impact of the gut microbiome on our health, much of today’s preventive work focuses on strategies to modulate either our microbiome or what we feed it through our diet.
In a study of type 2 diabetes (T2D) and low-grade inflammation, Tuomainen et al. identified an inverse association of the microbial metabolite indolepropionic acid (IPA) with T2D, suggesting a protective effect of IPA. Individuals with higher levels of this tryptophan derivative also reported greater dietary fiber consumption. This aligns with the understanding that dietary fiber influences gut microbiota composition and activity, potentially leading to increased IPA production. Elevated IPA levels were linked to reduced markers of low-grade inflammation, indicating potential anti-inflammatory effects of this metabolite (Tuomainen et al. 2018).
Building on these findings, interventions could explore:
- whether increasing the amount of fiber ingested reduces markers of inflammation and/or improves monitoring markers for patients with T2D,
- whether directly supplementation with IPA can lead to these effects.
Understanding the mechanistic link between these significant metabolic changes and health outcomes is a very valuable tool to determine the best course of action.
In a 2023 study, Tintelnot et al. identified another derivative of tryptophan through the activity of the microbiome: 3-indole acetic acid (3-IAA). Here, the metabolite was predictive of which patient would respond to chemotherapy treatment, likely due to its activity on the immune system; a story we will dive into more deeply in a future blog focused on predictive medicine.
Tryptophan metabolites have become a huge focus in inflammation and immunology research. Since tryptophan is an essential amino acid, and many of its derivatives are only present in human blood through metabolic activity in the microbiome, studying tryptophan pathways is fascinating and highly relevant. Find out more about tryptophan metabolism.
Probiotics
The more I learn about the microbiome, the more I realize how much we still don’t know. However, well-structured studies are steadily helping us understand what makes a “good” microbiome. And part of the answer lies not in which microbes are present, but in which metabolites they generate. Indeed, far from being deleterious, many microbial metabolites constitute the message that is sent to us and can contribute to our health.
A striking example of this comes in a 2019 paper by Wilmanski et al., which showed that a signature of 11 blood metabolites could accurately predict gut alpha-diversity with an AUC of 0.88. However, a battery of clinical tests and blood proteome analysis did not yield such results, failing to predict microbiome diversity. This study was a milestone to establish the relevance of measuring the metabolome in blood in the context of microbiome research.
Given the association of the gut microbiome with almost every chronic disease that plagues our societies, from neurodegenerative to autoimmune disease and cancer, modulating the composition and impact of the gut microbiome using probiotics is naturally a method of choice.
For example, in a mouse model of colitis, the introduction of Lactobacilli increased the microbial synthesis of indole-3-lactic acid (ILA), a precursor of IPA described previously (Wang et al. 2024). In a mouse model of depression, it is the strain Bifidobacterium breve that caused the production of ILA, thereby replenishing ILA stocks in the hippocampus (Qian et al. 2024).
Postbiotics
Although a diverse and stable microbiome is a key to good health, a logical next step is simply to supplement with the relevant beneficial metabolite. Many such postbiotic supplements exist, and short-chain fatty acids (SCFAs) are among the most promising ones. For example, butyrate acts as an energy source for enterocytes (the cells lining the intestine) and has been shown to promote and preserve gut health (reviewed by Ji et al. 2023). In clinical trials, butyrate supplementation led to significant improvements in body mass index (BMI) and insulin sensitivity in a cohort of obese children (Coppola et al. 2022).
In patients with liver steatosis and metabolic syndrome, a butyrate-based formula improved fatty liver index and reduced circulating levels of total cholesterol and total triglycerides (Fogacci et al. 2024).
Metabolic risk scores
Metabolomics can be used to assess a person’s health and calculate a score for the risk of developing disease. For instance, in a cohort of 21,323 individuals, serum metabolites and lipoproteins could identify patients with metabolic syndrome with an AUROC of 0.94 (Gil-Redondo et al. 2024).
In 2019, a study on 44,168 individuals rendered model using 14 circulating metabolites that could predict 5- and 10-year mortality with a C-statistic of 0.77 and 0.79, respectively (Deelen et al. 2019).
In 2024, Liu et al. combined metabolomics and genomics data in a metabolomics-based genome-wide association (mGWAS) study that identified 14 metabolites related to Alzheimer’s disease and found associations with microbiome-related features.
Outlook
In conclusion, metabolomics offers a wealth of information for a preventive approach to disease. Because our metabolism is sensitive to external factors, metabolomics is the ideal tool to:
- search for early markers of disease,
- calculate risk scores and follow their evolution through life,
- combine with the more static measure of the genome.
Sign up for our newsletter to receive an email when the next blog on 5P medicine comes out.
References
Coppola, S., et al.: Butyrate supplementation improves body mass index and insulin sensitivity in obese children (2022) Clinical Nutrition | https://doi.org/10.1016/j.clnu.2022.03.010
Deelen, J., et al.: A model using 14 circulating metabolites predicts 5- and 10-year mortality (2019) Nature Communications | https://doi.org/10.1038/s41467-019-11311-9
Fogacci, F., et al.: Butyrate-based formula improves fatty liver index and reduces cholesterol and triglyceride levels (2024) Journal of Clinical Lipidology | https://doi.org/10.1016/j.jacl.2024.01.005
Gil-Redondo, R., et al.: Serum metabolites and lipoproteins identify metabolic syndrome with AUROC 0.94 in a cohort of 21,323 individuals (2024) Metabolomics | https://doi.org/10.1007/s11306-024-02047-3
Ji, Y., et al.: The role of butyrate in promoting gut health (2023) Gut Microbes | https://doi.org/10.1080/19490976.2023.2234568
Liu, Y., et al.: Metabolomics-based genome-wide association study identifies metabolites related to Alzheimer’s disease and microbiome features (2024) Genome Medicine | https://doi.org/10.1186/s13073-024-01235-9
Pietzner, M., et al.: Metabolomics profiles associated with markers of inflammation (2017) Nature Medicine | https://doi.org/10.1038/nm.4333
Qian, Y., et al.: Bifidobacterium breve replenishes indole-3-lactic acid stocks in the hippocampus in a mouse model of depression (2024) Neuropsychopharmacology | https://doi.org/10.1038/s41386-024-01825-9
Tintelnot, S., et al.: 3-indole acetic acid predicts chemotherapy response through immune system activity (2023) Cancer Research | https://doi.org/10.1158/0008-5472.CAN-23-0142
Tuomainen, M., et al.: Indolepropionic acid inversely associated with type 2 diabetes and inflammation markers (2018) Diabetes Care | https://doi.org/10.2337/dc18-0980
Wang, J., et al.: Lactobacilli increase microbial synthesis of indole-3-lactic acid in a mouse model of colitis (2024) Microbiome | https://doi.org/10.1186/s40168-024-01586-w
Wilmanski, T., et al.: A signature of 11 blood metabolites predicts gut alpha-diversity with AUC 0.88 (2019) Cell Metabolism | https://doi.org/10.1016/j.cmet.2019.06.012


