- History & Evolution
- Biosynthesis vs. dietary uptake
- Kynurenine pathway
- Kynurenine metabolites ratios
- Kynurenine and the brain
- Kynurenine and microbiota
- Kynurenine and nutrition
- Clinical applications of kynurenine
- References
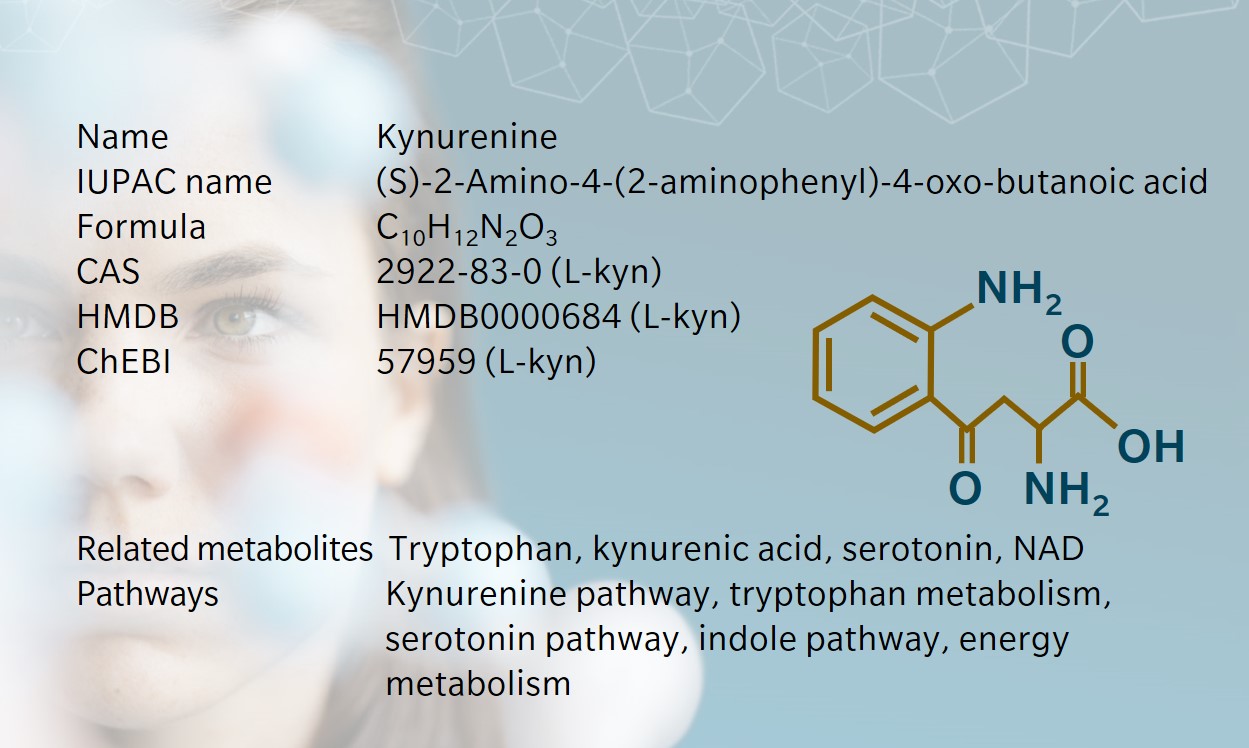
History & Evolution
1853: discovered | 1927: first isolated by Y. Kotake (Hayaishi 1993)
Kynurenine, a product of tryptophan metabolism, gets its name from the biofluid where it was first discovered: dog urine. In fact, the kynurenine pathway is found in many species, from microorganisms to plants and mammals. The pathway produces several neuroactive metabolites, as well as the cofactor NAD+.
Kynurenine pathway metabolites are linked to inflammation and the immune response, and have attracted interest recently as a potential target for treatments of neuroinflammatory disease.
Biosynthesis vs. dietary uptake
In humans, kynurenine is primarily synthesised endogenously from tryptophan. This reaction is catalysed by the enzyme tryptophan 2,3-dioxygenase (TDO) in the liver and, to a lesser extent, by indoleamine 2,3-dioxygenase (IDO) in other organs (Savitz 2020).
The level of kynurenine in the body is influenced by both the behaviour of these enzymes, and the tryptophan we get from our diet, for example in seeds, soybeans, dairy, meat, fish, eggs and legumes (Friedman 2018).
Tryptophan is the precursor to three major catabolic pathways: the kynurenine pathway, the serotonin pathway and the indole pathway (Taleb 2019). While kynurenine synthesis occurs in the liver, serotonin and indole are produced in the intestine before tryptophan enters the circulation.
Tryptophan molecules that are not degraded in the liver can also cross the blood-brain barrier, and then convert to serotonin or kynurenine by astrocytes or mesangial cells (Schwarcz et al. 2012).
Kynurenine pathway
The kynurenine pathway has two main branches, where kynurenine is converted to kynurenic acid by kynurenine aminotransferases (KAT), or to 3-hydroxyanthranillic acid by kynureninase and/or kynurenine 3-monooxygenase (KMO). These downstream metabolites are active in the nervous system and energy metabolism (Savitz 2020).
Kynurenine production can be triggered by signals from the immune system and inflammation, such as increased levels of tryptophan, hormonal signals such as cortisol, and inflammatory signals such as interleukins acting on IDO.
Kynurenine metabolites ratios
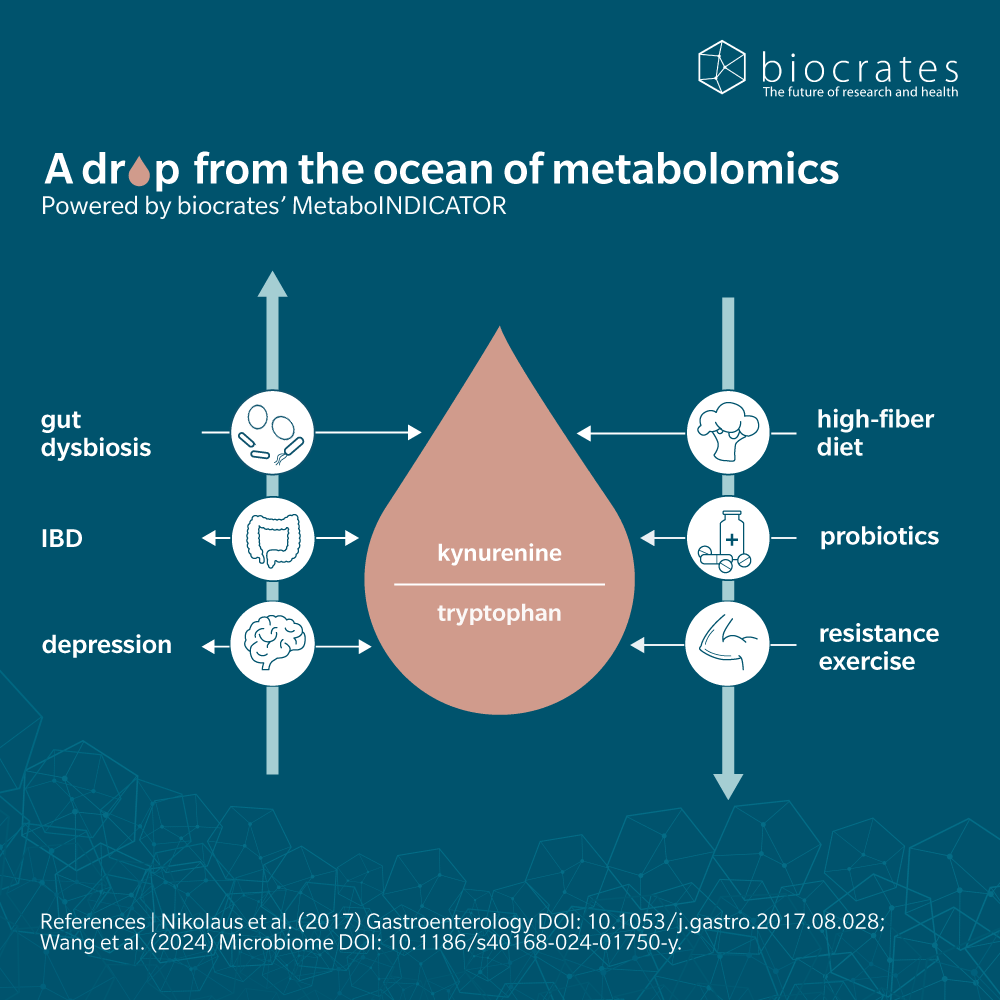
It’s often helpful to look at ratios of metabolites in a sample, rather than number.
For example, the kynurenine to tryptophan ratio is a useful proxy for the activity of enzymes involved in converting tryptophan to kynurenine. Although these ratios are no substitute for rigorous measurement of enzyme activity, they can provide a snapshot of the balance of a pathway and help uncover insights from metabolomic data.
In plasma, ratios of several kynurenine pathway metabolites may help to predict therapeutic response in fields as varied as gastroenterology (Nikolaus et al. 2017), neuroscience (Gulaj et al. 2010; Chang et al. 2018) and colon cancer (Steiner et al. 2018).
Kynurenine and the brain
The kynurenine pathway produces several neuroactive products within the brain – both neuroprotective and neurotoxic (Schwarcz et al. 2012). Again, ratios are helpful for the study of different neuroinflammatory diseases:
- Patients with Alzheimer’s disease (AD) will have lower levels of tryptophan and kynurenic acid and increased levels of quinolinic acid in their plasma, making it possible to differentiate AD patients from control patients (Gulaj et al. 2010)
- Patients with Parkinson’s disease (PD) will have increased ratios of kynurenic acid and kynurenic acid/kynurenine, with lower ratios of quinolinic acid and quinolinic acid/kynurenine acid (Chang et al. 2018)
- The ratio of kynurenine to tryptophan may be a predictor of remission in patients with major depression treated with celecoxib (Krause et al. 2017).
Kynurenine can also limit the tryptophan available for serotonin synthesis in the brain, though most serotonin is produced before dietary tryptophan is absorbed into the circulatory system in the intestine. (Kennedy et al. 2017).
Interestingly, within the intestinal region, serotonin was shown to act as an enterosyne having an important role in the regulation of the enteric nervous system (ENS) and the gut-brain axis (Abot et al. 2020).
Kynurenine and microbiota
Intestinal microbiota affect the kynurenine pathway by influencing the absorption of tryptophan before it reaches the liver, where most kynurenine synthesis takes place. In the intestinal lumen, bacteria can convert tryptophan to indole, sending dietary tryptophan to the indole pathway (Agus et al. 2018).
Indole itself serves as a signalling molecule for bacteria such as E. coli and can send warning signals for signs of inflammation in the gut. While downstream metabolites have been shown to challenge renal function (indole-sulfate), they also have beneficial anti-inflammatory and anti-oxidative properties for the host (indole-3-acetic acid, indole-3-propionic acid).
Kynurenine and nutrition
The effects of diet on health and disease are increasingly accepted. The ketogenic diet (low carbohydrates/high fat) has long been used to relieve epileptic patients from seizures and has shown interesting effects on metabolites of the kynurenine pathway (Żarnowska et al. 2019).
For example, in rats, a ketogenic diet was found to lower kynurenine levels in plasma and hippocampus tissue, and modulate other pathway metabolites (Heischmann et al. 2018).
When taken together with previous reports of quinolinic acid inducing seizures in mice and altered kynurenine-related metabolite levels in epilepsy models, there’s a clear argument for using such diets as therapy for epilepsy, via the kynurenine pathway (Sharma et al. 2015).
Clinical applications of kynurenine
Potential clinical applications of kynurenine are growing, especially in neuroscience, where the therapeutic inhibition of the kynurenine pathway is of particular interest. This could have important implications for the treatment of CNS infections, neuronal ischemic damage, and Huntington’s disease among others (reviewed by Stone et al. 2012)).
Learn more about the roles of kynurenine and its derivatives in complex chronic diseases such as cancer, Alzheimer’s disease, depression, inflammatory bowel disease, multiple sclerosis and diabetes in our whitepaper “Complex chronic diseases have a common origin”.
References
Abot A, Wemelle E, Laurens C, Paquot A, Pomie N, Carper D, Bessac A, Orea XM, Fremez C, Fontanie M, Lucas A, Lesage J, Everard A, Meunier E, Dietrich G, Muccioli GG, Moro C, Cani PD, Knauf C: Identification of new enterosynes using prebiotics: roles of bioactive lipids and mu-opioid receptor signalling in humans and mice (2020) Gut. | http://dx.doi.org/10.1136/gutjnl-2019-320230
Agus A, Planchais J, Sokol H (2018): Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease (2018) Cell host & microbe | https://doi.org/10.1016/j.chom.2018.05.003
Campbell BM, Charych E, Lee AW; Möller T: Kynurenines in CNS disease: regulation by inflammatory cytokines (2014) Frontiers in neuroscience | https://doi.org/10.3389/fnins.2014.00012
Chang KH, Cheng ML, Tang HY, Huang CY, Wu YR, Chen CM: Alternations of Metabolic Profile and Kynurenine Metabolism in the Plasma of Parkinson’s Disease (2018) Molecular neurobiology | https://doi.org/10.1007/s12035-017-0845-3
Friedman M: Analysis, Nutrition, and Health Benefits of Tryptophan (2018) International journal of tryptophan research | https://doi.org/10.1177/1178646918802282
Fukui S, Schwarcz R, Rapoport SI, Takada Y, Smith QR: Blood-brain barrier transport of kynurenines: implications for brain synthesis and metabolism (1991) Journal of neurochemistry | https://doi.org/10.1111/j.1471-4159.1991.tb03460.x
Gulaj E, Pawlak K, Bien B, Pawlak D: Kynurenine and its metabolites in Alzheimer’s disease patients (2010) Advances in medical sciences | https://doi.org/10.2478/v10039-010-0023-6
Hayaishi O: My life with tryptophan–never a dull moment (1993) Protein science | https://doi.org/10.1002/pro.5560020320
Heischmann S, Gano LB, Quinn K, Liang LP, Klepacki J, Christians U: Regulation of kynurenine metabolism by a ketogenic diet (2018) Journal of lipid research | https://doi.org/10.1194/jlr.M079251
Kennedy PJ, Cryan JF, Dinan TG, Clarke G: Kynurenine pathway metabolism and the microbiota-gut-brain axis (2017) Neuropharmacology | https://doi.org/10.1016/j.neuropharm.2016.07.002
Krause D, Myint AM, Schuett C, Musil R, Dehning S, Cerovecki A et al.: High Kynurenine (a Tryptophan Metabolite) Predicts Remission in Patients with Major Depression to Add-on Treatment with Celecoxib (2017) Frontiers in psychiatry | https://doi.org/10.3389/fpsyt.2017.00016
Nikolaus S, Schulte B, Al-Massad N, Thieme F, Schulte DM, Bethge J et al.: Increased Tryptophan Metabolism Is Associated With Activity of Inflammatory Bowel Diseases (2017) Gastroenterology https://doi.org/10.1053/j.gastro.2017.08.028
Savitz J: The kynurenine pathway: a finger in every pie (2020) Molecular psychiatry | https://doi.org/10.1038/s41380-019-0414-4
Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ: Kynurenines in the mammalian brain: when physiology meets pathology (2012) Nature reviews. Neuroscience | https://doi.org/10.1038/nrn3257
Sharma M, Anand C, Chugani DC: Role of the Kynurenine Pathway in Epilepsy. In Sandeep Mittal (Ed.): Targeting the Broadly Pathogenic Kynurenine Pathway (2015) Springer International Publishing | https://doi.org/10.1007/978-3-319-11870-3_16
Steiner N, Müller U, Hajek R, Sevcikova S, Borjan B, Jöhrer K et al.: The metabolomic plasma profile of myeloma patients is considerably different from healthy subjects and reveals potential new therapeutic targets (2018) PloS one | https://doi.org/10.1371/journal.pone.0202045
Stone TW, Forrest CM, Darlington GL: Kynurenine pathway inhibition as a therapeutic strategy for neuroprotection (2012) The FEBS journal | https://doi.org/10.1111/j.1742-4658.2012.08487.x
Taleb S: Tryptophan Dietary Impacts Gut Barrier and Metabolic Diseases (2019) Frontiers in immunology| https://doi.org/10.3389/fimmu.2019.02113
Żarnowska I, Wróbel-Dudzińska D, Tulidowicz-Bielak M, Kocki T, Mitosek-Szewczyk K, Gasior M, Turski WA: Changes in tryptophan and kynurenine pathway metabolites in the blood of children treated with ketogenic diet for refractory epilepsy (2019) Seizure | https://doi.org/10.1016/j.seizure.2019.05.006