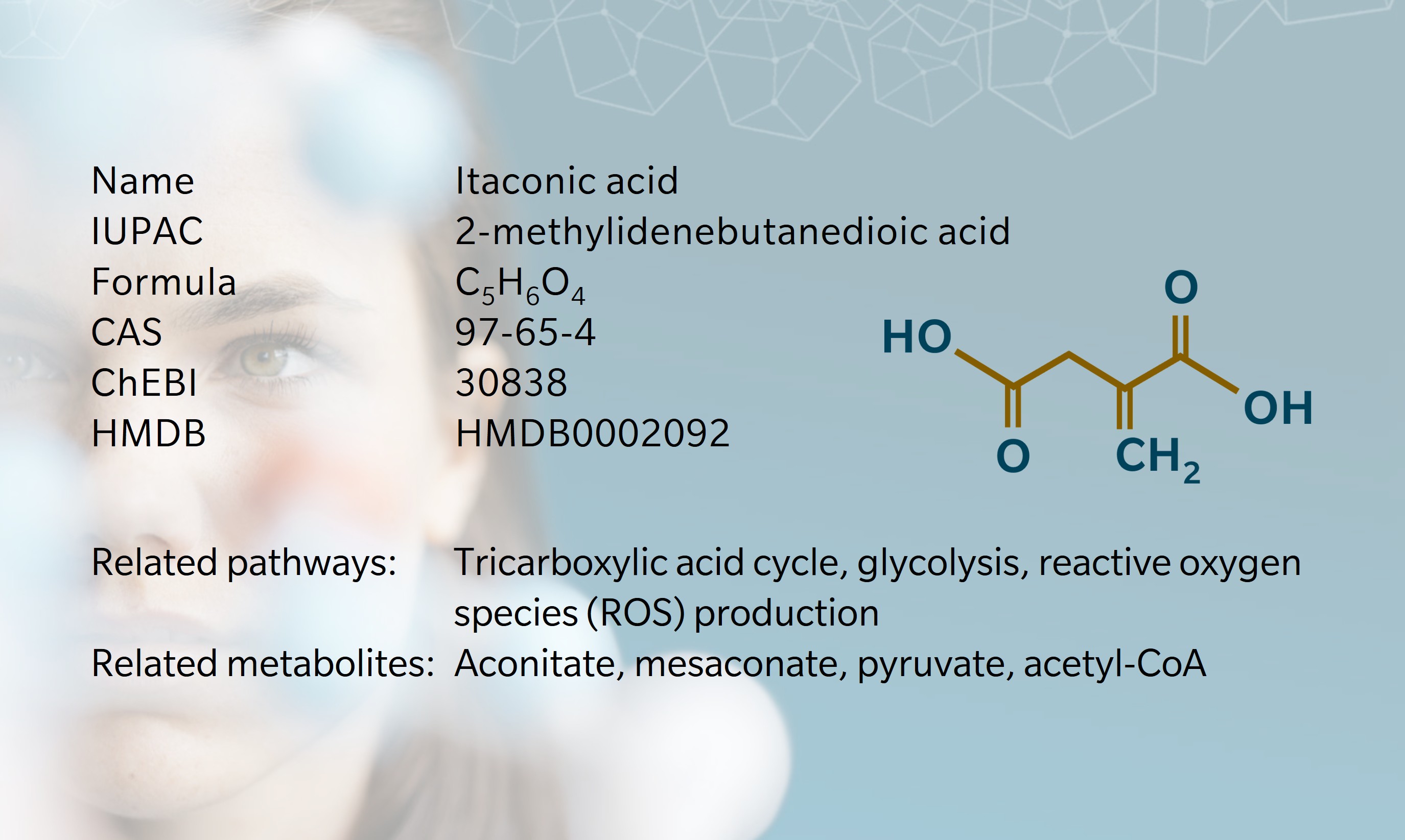
- History & Evolution
- Biosynthesis & dietary uptake
- Itaconic acid and the microbiome
- Itaconic acid, immunity and inflammation
- Itaconic acid and cardiovascular disease
- Itaconic acid and neurology
- Itaconic acid and oncology
- Itaconic acid and 5P medicine
- References
History & Evolution
1836: discovery (Baup, 1836) | 1840: first synthesis (Crasso, G., 1840) | 1960s: first industrial applications | 2012: first synthesized in mammalian cells (Sugimoto, et al., 2011)
In 1836, Swiss chemist Samuel Baup discovered an unknown compound when experimenting with the distillation of citric acid (Baup, 1836). In 1840, Gustav Crasso synthesized the same compound through the decarboxylation of aconitate, and called it itaconate – an anagram of its precursor (Crasso, 1840). This unsaturated dicarboxylic acid received little attention for almost a hundred years, until 1931, when Japanese mycologist Kinoshita discovered a strain of Aspergillus fungi in salted prune juice that produced the metabolite (Kinoshita, 1932). He named the fungus Aspergillus itaconicus.
By the 1960s, itaconic acid had caught the attention of the polymer industry, thanks to the useful double bond of its methylene group. Now, around 40,000 tons are produced each year, primarily synthesized from Aspergillus terreus (Cordes, 2015).
Scientific interest in itaconate’s biological role was renewed in the early 2010s, when Sugimoto et al. discovered that it could be synthesized in mammalian immune cells (Sugimoto et al., 2011). Since then, itaconic acid’s role as a key player in immunometabolism and inflammatory regulation has become an active area of research (Diotallevi et al., 2021).
Biosynthesis vs. dietary uptake
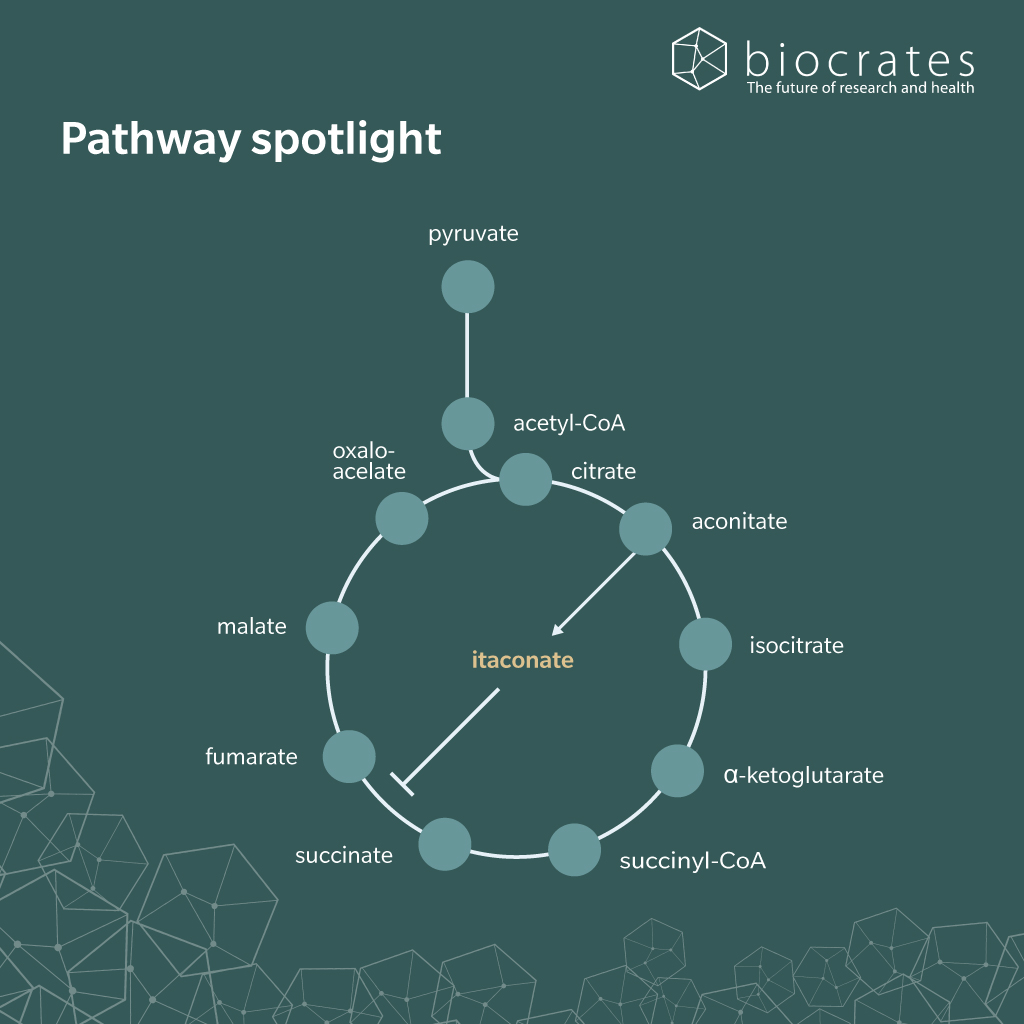
In humans, itaconic acid is an endogenous metabolite synthesized primarily in the mitochondria of activated macrophages (Peace et al., 2022). It is synthesized by decarboxylating cis-aconitate, an intermediate of the tricarboxylic acid (TCA) cycle. Metabolomics has shown that this occurs through the action of the enzyme cis-aconitase decarboxylase, also known as immune-responsive gene 1 (IRG1 or ACOD1) (Michelucci et al., 2013). Expression of IRG1 is upregulated during infection and inflammation, leading to increased itaconate production. Plasma concentrations are therefore highly variable, depending on immune status.
Humans do not obtain itaconic acid directly from the diet, but dietary factors may affect its production. For example, carbohydrate intake affects the TCA cycle and energy metabolism, which in turn influences the availability of itaconate precursors like cis-aconitate. Under inflammatory conditions, nutrient availability may affect itaconate synthesis.
Itaconate is the biologically active, deprotonated form of itaconic acid, which contains two carboxylic acid groups that lose protons at pH levels above 7. This gives itaconate a double negative charge and makes it the dominant form found in the bloodstream. Structurally, it resembles other dicarboxylates such as succinate, malonate, phosphoenolpyruvate and fumarate (Peace et al., 2022).
Itaconic acid and the microbiome
While itaconic acid is synthesized by environmental microbes, like some Aspergillus species, it does not appear to be produced by gut microbiota in significant amounts. That said, it has been described as a “microbiota-associated metabolite” because its production in the mitochondria of host immune cells is triggered by microbial components such as lipopolysaccharide (LPS) from Gram-negative bacteria (Fedotcheva et al., 2022).
A study using integrated multiomics approaches showed that altered itaconate metabolism leads to significant changes in gut microbiota composition. Using a mouse model, Eberhart et al. (2024) found that in response to a high-fat diet, the itaconate-synthesizing enzyme ACOD1 promotes gut dysbiosis associated with obesity and inflammation, while genetic deletion of ACOD1 protects against metabolic disease (Eberhart et al., 2024). In addition, fecal metagenomics and microbiota transplantation showed that itaconate inhibits growth of Bacteroidaceae. These findings suggest that itaconate plays a role in diet-induced obesity and positions the ACOD1–itaconate pathway as a potential therapeutic target for the inflammatory effects of obesity.
Itaconic acid also inhibits bacterial isocitrate lyase, a key enzyme in the glyoxylate shunt during bacterial infection (Peace, 2022). This limits the growth of pathogens like Mycobacterium tuberculosis, Pseudomonas indigofera and Salmonella. However, some bacteria have developed strategies to protect themselves against itaconate’s antimicrobial properties (Peace, 2022). For example, Pseudomonas aeruginosa, Yersinia pestis and M. tuberculosis can break down itaconate into pyruvate and acetyl-CoA, while P. aeruginosa and Staphylococcus aureus respond to itaconate-induced stress by shifting from LPS to extracellular polysaccharide production, leading to biofilm formation that provides intracellular protection for the bacteria.
See how the MxP® Quant 1000 assay helps scientists investigate the impact of energy metabolism on health by accurately profiling TCA metabolites with targeted metabolomics
Itaconic acid, immunity and inflammation
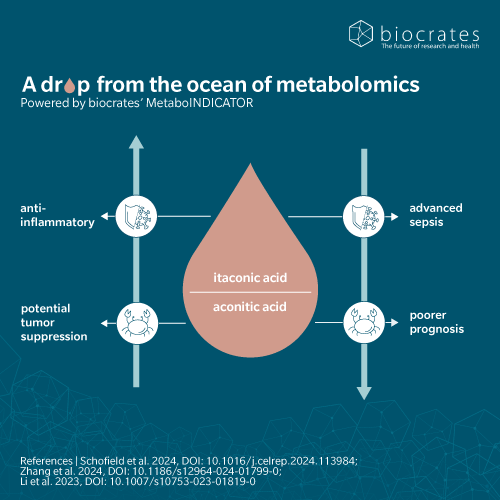
Itaconic acid plays an important role in immune regulation and inflammation through the antibacterial mechanisms mentioned above. It also modulates the production of reactive oxygen species (ROS), which helps defend against bacterial and viral infections. Itaconate’s antiviral properties have been found to inhibit Zika virus replication (Daniels et al., 2019).
Beyond its antimicrobial activity, itaconic acid also acts as an anti-inflammatory metabolite by targeting key metabolic pathways in activated macrophages. It inhibits succinate dehydrogenase, a key enzyme in the TCA cycle, which alters mitochondrial metabolism and promotes anti-inflammatory cytokine production (Cordes et al., 2016). It also disrupts mitochondrial energy metabolism by disrupting oxidative phosphorylation and fatty acid oxidation. Recent studies have shown that itaconic acid can promote the pentose phosphate pathway, increasing nicotinamide adenine dinucleotide phosphate (NADPH) levels and enhancing NADPH oxidase-mediated ROS production. These ROS contribute to the expression of anti-inflammatory genes, restrict pathogens like Salmonella typhimurium and reduce pro-inflammatory cytokine production (Zhu et al., 2021).
These findings have led to broader recognition of the itaconate pathway in immune regulation. A review by Li et al. (2020) describes the IRG1/itaconate axis as a central feature in immunometabolic regulation, supported by metabolomics data that link itaconate to changes in mitochondrial respiration, oxidative stress and inflammatory signaling in macrophages (Li et al., 2020).
Given these properties, itaconic acid has emerged as a metabolite of interest in immune-mediated diseases. A recent review by Luo et al. (2024) suggests itaconate and its derivatives may play a role in conditions such as rheumatoid arthritis, multiple sclerosis , type 1 diabetes mellitus and autoimmune hepatitis (Luo et al., 2025).
Itaconic acid and cardiovascular disease
Research shows that itaconate may have a protective effect in cardiovascular disease. In models of atherosclerosis, IRG1 deficiency has been shown to increase plaque burden (Harber et al., 2024), while treatment with 4-octyl itaconate (4-OI) reversed plaque inflammation in mice and reduce cardiovascular inflammation in human immune cells (Cyr et al., 2024).
Itaconate also supports cardiac repair after myocardial infarction. A study using targeted metabolomics found that after efferocytosis (the clearing of dead cells which is essential for cardiac repair), altered signaling in macrophages expressing the TREM2 receptor disrupted the TCA cycle (Gong et al., 2024). This resulting increase in itaconate production reduced cardiomyocyte apoptosis and promoted fibroblast proliferation. Increased TREM2 levels led to improved cardiac function.
Itaconic acid and neurology
Neuroinflammation is a well-established driver in the progression of numerous neurological disorders, including Alzheimer’s disease (AD), Parkinson’s disease (PD), multiple sclerosis (MS) and stroke. Itaconate and its derivatives may help counteract this neuroinflammatory activity in the central nervous system through the regulation of immune pathways including Nrf2/KEAP1, ROS production and the NLRP3 inflammasome (Kong et al., 2024).
In LPS-induced neuroinflammation, both itaconate and the related isomer mesaconate have been found to reduce inflammation in the brain and improve synaptic plasticity, linked to memory and cognitive processes (Ohm et al., 2024). A study using untargeted serum metabolomics in PD patients also identified reduced levels of circulating itaconate, suggesting a role in disease-associated immune dysregulation (Paul et al., 2023).
These findings suggest that itaconate may be a promising candidate as a biomarker and therapeutic target in neurological conditions.
Itaconic acid and oncology
Itaconic acid has also been implicated in cancer biology. In peritoneal tumors such as melanoma and ovarian carcinoma, metabolomics techniques have shown an upregulation of itaconic acid (Weiss et al., 2018). Tumor-associated macrophages were found to accumulate itaconate, resulting in increased oxidative phosphorylation and mitochondrial ROS production, and in turn promoting tumor growth. Silencing IRG1 was found to reduce the tumor burden.
However, there is also evidence that itaconic acid has anti-tumor effects (Perkovic et al., 2020) (Wang, Z. et al., 2024). A mouse model showed that dimethyl itaconate suppresses colitis-associated colorectal cancer (Wang et al., 2020). In another animal study, 4-OI was found to induce ferroptosis in the treatment of retinoblastoma (Liu et al., 2022).
Thes findings suggest that more research is needed to fully understand the role of itaconic acid in different types of cancer.
Itaconic acid and 5P medicine
In the context of 5P medicine – predictive, preventive, precision, population-based, participatory medicine – itaconic acid is again relevant due to its role in mediating immune responses and inflammation, both of which contribute to many prevalent chronic diseases. A white paper by biocrates, “Chronic diseases have a common origin”, highlights the underlying role of inflammation in complex chronic disease.
Obesity is a major public health concern and key target for preventive strategies. Dietary itaconate has been shown to reduce visceral fat accumulation in rats, highlighting a potential role in early intervention and metabolic regulation (Sakai et al., 2004).
As a biomarker of disease, itaconate levels in blood or immune cells may signal early inflammatory activity or therapeutic response, as seen in conditions like rheumatoid arthritis (Daly et al., 2020), non-alcoholic fatty liver disease (Weiss et al., 2018) and bacterial infections (Singh et al., 2021). In each of these examples, metabolomics has played a role in providing insights about the presence and behavior of this important metabolite.
Itaconic acid is a promising focus for future research, with omics technologies offering a powerful tool to support 5P medicine.
References
Baup, S.: Ueber eine neue Pyrogen-Citronensäure, und über Benennung der Pyrogen-Säuren überhaupt. (1836) Ann. Pharm | https://doi.org/10.1002/jlac.18360190107.
Cordes, T. et al.: Itaconic Acid: The Surprising Role of an Industrial Compound as a Mammalian Antimicrobial Metabolite. (2015) Annual Review of Nutrition | https://doi.org/10.1146/annurev-nutr-071714-034243.
Cordes, T. et al.: Immunoresponsive Gene 1 and Itaconate Inhibit Succinate Dehydrogenase to Modulate Intracellular Succinate Levels. (2016) J Biol Chem.| https://doi.org/10.1074/jbc.M115.685792.
Crasso, G.: Untersuchungen über das Verhalten der Citronsäure in höherer Temperatur und die daraus hervorgehenden Produkte (1840) Ann. Chem. Pharm. | https://doi.org/10.1002/jlac.18400340104.
Cyr, Y. et al.: The IRG1–itaconate axis protects from cholesterol-induced inflammation and atherosclerosis. (2024) Natl Acad Sci USA | https://doi.org/10.1073/pnas.2400675121.
Daly, R. et al.: Changes in Plasma Itaconate Elevation in Early Rheumatoid Arthritis Patients Elucidates Disease Activity Associated Macrophage Activation. (2020) Metabolites | https://doi.org/10.3390/metabo10060241.
Daniels, B. et al.: The nucleotide sensor ZBP1 and kinase RIPK3 induce the enzyme IRG1 to promote an antiviral metabolic state in neurons.(2019) Immunity | https://doi.org/10.1016/j.immuni.2018.11.017.
Diotallevi, M. et al.: Itaconate as an inflammatory mediator and therapeutic target in cardiovascular medicine. (2021) Biochem Soc Trans, | https://doi.org/10.1042/BST20210269.
Eberhart, T. et al.: ACOD1 deficiency offers protection in a mouse model of diet-induced obesity by maintaining a healthy gut microbiota. (2024) Cell Death Dis. | https://doi.org/10.1038/s41419-024-06483-2.
Fedotcheva, N. et al.: Influence of Microbial Metabolites and Itaconic Acid Involved in Bacterial Inflammation on the Activity of Mitochondrial Enzymes and the Protective Role of Alkalization.(2022) Int J Mol Sci. | https://doi.org/10.3390/ijms23169069.
Gong, S. et al.: TREM2 macrophage promotes cardiac repair in myocardial infarction by reprogramming metabolism via SLC25A53. (2024) Death Differ. | https://doi.org/10.1038/s41418-023-01252-8.
Harber, K. et al.: Targeting the ACOD1-itaconate axis stabilizes atherosclerotic plaques. (2024) Biol. | https://doi.org/10.1016/j.redox.2024.103054.
Kinoshita, K.: Über die Produktion von Itaconsäure und Mannit durch einen neuen Schimmelpilz, Aspergillus itaconicus. (1932) Acta Phytochimica | https://www.scirp.org/reference/referencespapers?referenceid=899199
Kong, X. et al.: The anti-inflammatory effects of itaconate and its derivatives in neurological disorders. (2024) Cytokine & Growth Factor Reviews, | https://doi.org/10.1016/j.cytogfr.2024.07.001.
Li, R. et al.: Itaconate: A Metabolite Regulates Inflammation Response and Oxidative Stress. (2020) Oxid Med Cell Longev.| https://doi.org/10.1155/2020/5404780.
Liu, K. et al.: Induction of autophagy-dependent ferroptosis to eliminate drug-tolerant human retinoblastoma cells. (2022) Death Dis. | https://doi.org/10.1038/s41419-022-04974-8.
Luo, Y. et al.: Metabolic Regulation of Inflammation: Exploring the Potential Benefits of Itaconate in Autoimmune Disorders. (2025) Immunology | https://doi.org/10.1111/imm.13875.
Michelucci, A. et al.: Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production. (2013) PNAS | https://doi.org/10.1073/pnas.1218599110.
Ohm, M. et al.: The potential therapeutic role of itaconate and mesaconate on the detrimental effects of LPS-induced neuroinflammation in the brain. (2024) J Neuroinflammation | https://doi.org/10.1186/s12974-024-03188-3.
Paul, K. et al.: Untargeted serum metabolomics reveals novel metabolite associations and disruptions in amino acid and lipid metabolism in Parkinson’s disease. (2023) Mol Neurodegener. | https://doi.org/10.1186/s13024-023-00694-5.
Peace, C. et al.: The role of itaconate in host defense and inflammation. (2022) J Clin Invest. | https://doi.org/10.1172/JCI148548.
Perkovic, I. et al.: Itaconic acid hybrids as potential anticancer agents. (2020) Mol Divers. | https://doi.org/10.1007/s11030-020-10147-6.
Sakai, A. et al.: Itaconate reduces visceral fat by inhibiting fructose 2,6-bisphosphate synthesis in rat liver. (2004) Nutrition | https://doi.org/10.1016/j.nut.2004.08.007.
Singh, S. et al.: Integrative metabolomics and transcriptomics identifies itaconate as an adjunct therapy to treat ocular bacterial infection. (2021) Rep Med, | https://doi.org/10.1016/j.xcrm.2021.100277.
Sugimoto, M. et al.: Non-targeted metabolite profiling in activated macrophage secretion. (2011) Metabolomics | https://doi.org/10.1007/s11306-011-0353-9.
Wang, Q. et al.: The anti-inflammatory drug dimethyl itaconate protects against colitis-associated colorectal cancer. (2020) Journal of Molecular Medicine | https://doi.org/10.1007/s00109-020-01963-2.
Wang, Z. et al.: Cancer cell-intrinsic biosynthesis of itaconate promotes tumor immunogenicity. (2024) EMBO J. | https://doi.org/10.1038/s44318-024-00217-y.
Weiss, J. et al.: Itaconic acid mediates crosstalk between macrophage metabolism and peritoneal tumors. (2018) J Clin Invest, | https://doi.org/10.1172/JCI99169.
Zhu, X. et al.: Itaconic acid exerts anti-inflammatory and antibacterial effects via promoting pentose phosphate pathway to produce ROS. (2021) Sci Rep. | https://doi.org/10.1038/s41598-021-97352-x.