History & evolution
Biosynthesis vs. dietary uptake
α-AAA and cardiometabolic diseases
α-AAA and neurology
α-AAA and cancer
α-AAA, intestinal dysbiosis, and immunity
α-AAA and aging
α-AAA as a metabotoxin
History and evolution
1945: first report of α-AAA (Machebouef et al.1945) | 1948: discovery of α-AAA’s role in lysine metabolism (Borsook et al. 1948) | 1954: discovery of α-AAA’s mechanism of action in lysine metabolism (Rothstein et al. 1954)
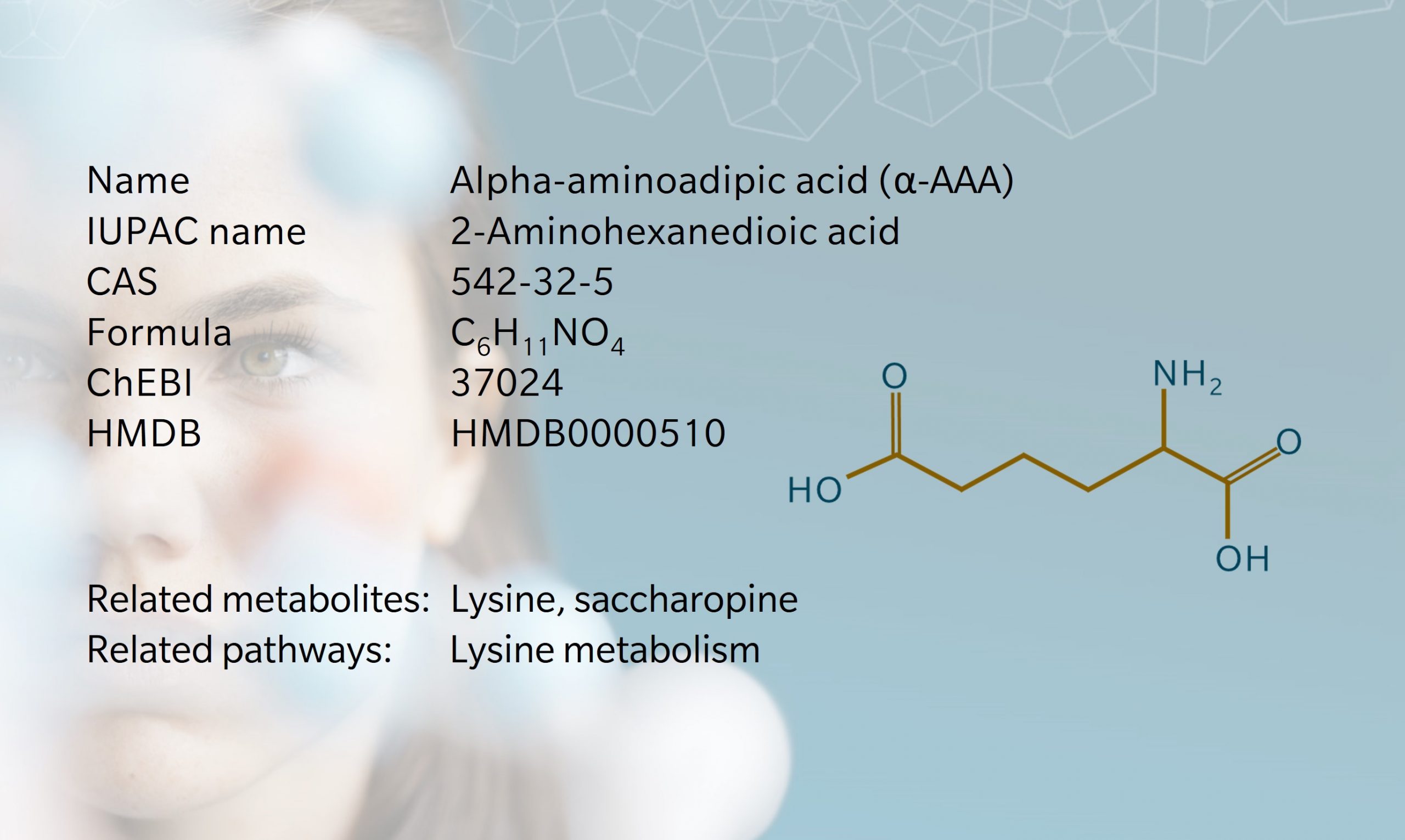
Aminoadipic acid is an alpha amino acid, meaning its amino group is attached to the carbon atom in the alpha position. It’s a nonproteinogenic amino acid found in all eukaryotes.
Discovery of α-AAA had a somewhat patchy start: Blass and Macheboeuf first reported its presence in biological material in 1945, after claiming to isolate it from cholera Vibrio (Machebouef et al.1945). However, they retracted the claim after re-running their experiment. In 1948, Borsook et al. found that the retraction by Blass and Macheboeuf was based on a paper chromatography method that did not differentiate α-AAA and glutamic acid, and the presence of α-AAA in cholera was subsequently confirmed. Around the same time, Neuberger and Sanger identified α-AAA as a product of lysine degradation in mammals (Borsook et al. 1948).
In humans, α-AAA is involved in the lysine and saccharopine pathways. It has been shown to act as an acidogen, diabetogen, atherogen and metabotoxin, making it a metabolite of interest in diseases including cancer, diabetes and cardiovascular disease (Human Metabolome Database 2022). In fungi, α-AAA is a precursor to lysine and penicillin. The α-aminoadipate pathway has been explored as a potential target for antifungal drug therapies (Fazius et al. 2012).
Biosynthesis vs. dietary uptake
In humans, α-AAA is primarily obtained sourced from endogenous synthesis rather than dietary uptake. It is formed through the catabolism of lysine. As an essential amino acid, lysine cannot be synthesized in the body and must be obtained through the diet (Matthews 2020). Lysine itself is found in high-protein foods such as meat, dairy products, eggs and legumes.
Once ingested, lysine can be metabolized by various enzymatic reactions. The primary pathway begins with the formation of α-AAA (Matthews 2020). This metabolite is derived from α-ketoadipic acid through the enzymatic conversion of α-aminoadipic semialdehyde by α-aminoadipic semialdehyde dehydrogenase. This α-AAA can serve as a precursor for the saccharopine pathway, which branches off the lysine pathway. Saccharopine is produced when α-AAA is combined with glutamate. In reverse, saccharopine metabolism results in α-AAA and glutamate, contributing to lysine synthesis. This occurs primarily in the liver and kidneys. α-AAA is then converted to acetyl coenzyme-A in the mitochondria, contributing to multiple biochemical reactions throughout the body.
In the brain, lysine catabolism occurs via the pipecolate pathway. The two pathways converge at the level of α-AAA (in the saccharopine pathway) and δ-piperideine-6-carboxylate (a downstream metabolite in the pipecolate pathway) (Hallen et al. 2013).
α-AAA and cardiometabolic diseases
While α-AAA is relatively understudied, it has attracted attention as a potential biomarker of type 2 diabetes. A 2013 case-control study used a metabolomics platform to analyze metabolite concentrations in individuals who developed diabetes (Wang et al. 2013). In this study, α-AAA was the metabolite most strongly associated with risk of developing diabetes. People with α-AAA concentrations in the top quartile were four times more likely to develop diabetes. However, α-AAA levels did not correlate with other diabetes biomarkers, suggesting a distinct pathophysiological pathway. Experimental studies in mice from the same article suggested that administering α-AAA could reduce fasting plasma glucose levels and enhance insulin secretion, further indicating its role as a biomarker of diabetes risk and modulator of glucose homeostasis.
Metabolomics was also used to study elderly individuals, with reference to lipid disorders. In this context, α-AAA levels were found to be elevated in those with lipid disorders (Lo et al. 2019). Several additional studies have used metabolomics to investigate the relationship between α-AAA and obesity-related factors including insulin resistance and metabolic disorders and found a positive correlation (Lee et al. 2019) (Gao et al. 2016) (Guevara-Cruz et al. 2018).
A 2019 study found that α-AAA treatment reduced body weight and fat accumulation and improved fasting glucose levels in mice with diet-induced obesity (Xu et al. 2019). This further suggests that α-AAA could be a potential therapeutic strategy for treatment of diabetes and obesity. The mechanism of action in cardiometabolic disease is thought to relate to DHTKD1, a gene involved in the α-AAA pathway (Wang et al. 2021).
α-AAA and neurology
Changes in α-AAA levels have been observed in neurological conditions such as Alzheimer’s disease (AD), again using metabolomics. One study found that α-AAA concentrations in fasting serum samples changed in symptomatic stages of AD (Toledo et al. 2017). This suggests that α-AAA could play a role in the diagnosis and prognosis of AD. Its potential as a marker for amnestic mild cognitive impairment has also been suggested (Wang et al. 2014). Aminoadipic acid has been shown to act on the neuronal N-methyl-D-aspartate (NMDA) receptor (Human Metabolome Database 2022)
A metabolomics study using urine samples found that alpha-aminoadipic semialdehyde, a derivative of α-AAA, could be a potential biomarker for pyridoxine-dependent epilepsy (PDE) (Ferrer-López et al. 2014). It also suggested that elevated urinary levels of the metabolite could confirm other metabolic disorders, such as molybdenum cofactor and isolated sulphite oxidase deficiencies. An observational study suggested that a lysine-restricted diet could reduce potentially neurotoxic biomarkers (such as α-aminoadipic semialdehyde) and improve developmental outcomes in children with PDE (van Karnebeek et al. 2012).
α-AAA and cancer
Higher levels of certain amino acids, including aminoadipic acid, have been associated with a reduced risk of advanced stage head and neck cancers (Cadoni et al. 2020). An investigation into the diagnostic and prognostic potential of metabolites in prostate cancer found higher levels of α-AAA in malignant tissues compared to non-malignant tissue (Jung et al. 2013). α-AAA levels were also associated with biochemical tumor recurrence. This suggests that α-AAA could serve as a potential biomarker for prostate cancer, combined with traditional clinicopathological factors.
α-AAA, intestinal dysbiosis, and immunity
As with many metabolites, α-AAA plays a role in gut health, with implications for broader health and disease. Chronic inflammation of the gut can affect immunity, with effects seen throughout the body. For example, research has shown a correlation between levels of Collinsella and α-AAA, and the role of Collinsella in altering intestinal permeability in patients with rheumatoid arthritis (Mena-Vázquez et al. 2020).
α-AAA and aging
Several studies point to a link between aminoadipic acid and age-related deterioration in skin and eyes. Lysine residues in older skin proteins can undergo oxidative deamination as they age, resulting in α-AAA and allysine (Sell et al. 2007). The same study measured levels of allysine and α-AAA in insoluble human skin collagen from subjects aged 10-90 years old. α-AAA levels increased with age, while allysine remained constant. The former increased even more in individuals with diabetes, renal failure, or sepsis.
A similar pattern was observed in a study that looked at oxidative deamination of lysine residues in the aging human lens (Fan et al. 2008). The exact mechanism of action was unclear but thought to involve catalytic metals. Oxidation of allysine to α-AAA was modest in lens compared to skin.
α-AAA as a metabotoxin
Metabotoxins are endogenous metabolites that can trigger health problems if concentrations become too high for too long. Chronically high levels of aminoadipic acid have been linked to two inborn errors of metabolism: 2-aminoadipic aciduria and 2-oxoadipic aciduria (Danhauser et al. 2012). These are associated with DHTKD1 mutations. These conditions are very rare, and while some individuals are asymptomatic, others experience developmental delay, cognitive limitations, hypotonia, ataxia and epilepsy (Danhauser et al. 2012).
References
Borsook, H. et al.: The degradation of L-lysine in guinea pig liver homogenate: formation of alpha aminoadipic acid. (1948) Journal of Biological Chemistry | https://authors.library.caltech.edu/11807/
Cadoni, G. et al.: Prognostic Role of Serum Amino Acids in Head and Neck Cancer. (2020) Dis Markers | https://doi.org/10.1155/2020/2291759
Danhauser, K. et al.: DHTKD1 mutations cause 2-aminoadipic and 2-oxoadipic aciduria. (2012) Am J Hum Genet | https://doi.org/10.1016/j.ajhg.2012.10.006
Fan, X. et al.: Allysine and 2-Aminoadipic Acid Are Markers of Protein Carbonyl Oxidation in Aging Human Lens. (2008) Invest. Ophthalmol. Vis. Sci.
Fazius, F. et al.: The fungal α-aminoadipate pathway for lysine biosynthesis requires two enzymes of the aconitase family for the isomerization of homocitrate to homoisocitrate. (2012) Mol Microbiol | https://doi.org/10.1111/mmi.12076
Ferrer-López, I. et al.: Determination of urinary alpha-aminoadipic semialdehyde by LC-MS/MS in patients with congenital metabolic diseases. (2014) J Chromatogr B Analyt Technol Biomed Life Sci | https://doi.org/10.1016/j.jchromb.2013.10.032
Gao, X. et al.: Serum metabolic biomarkers distinguish metabolically healthy peripherally obese from unhealthy centrally obese individuals. (2016) Nutr Metab | https://doi.org/10.1186/s12986-016-0095-9
Guevara-Cruz, M. et al.: Amino acid profiles of young adults differ by sex, body mass index and insulin resistance. (2018) Nutr Metab Cardiovasc Dis, | https://doi.org/10.1016/j.numecd.2018.01.001
Hallen, A. et al.: Lysine metabolism in mammalian brain: an update on the importance of recent discoveries. (2013) Amino Acids | https://doi.org/10.1007/s00726-013-1590-1
Human Metabolome Database: Metabocard for aminoadipic acid (HMDB0000510) HMDB 5.0: the Human Metabolome Database for 2022. (2022) Nucleic Acids Res. | https://doi.org/10.1093/nar/gkab1062
Jung. K. et al.: Tissue metabolite profiling identifies differentiating and prognostic biomarkers for prostate carcinoma. (2013) Int J Cancer | https://doi.org/10.1002/ijc.28303
Lee, H.J. et al.: 2-Aminoadipic acid (α-AAA) as a potential biomarker for insulin resistance in childhood obesity. (2019)Sci Rep | https://doi.org/10.1038/s41598-019-49578-z
Lo, C.J. et al.: Metabolic Signature Differentiated Diabetes Mellitus from Lipid Disorder in Elderly Taiwanese. (2019) J Clin Med, | https://doi.org/10.3390/jcm8010013
Machebouef, M. et al.: On the existence, in choleric vibrios, of new amino acids extractable by acetone and by methyl alcohol; identification of one of them, aminoadipic acid [in French] (1945) C R Hebd Seances Acad Sci
Matthews, D.: Review of Lysine Metabolism with a Focus on Humans. (2020) J Nutr, 150(Suppl 1) | https://doi.org/10.1093/jn/nxaa224
Mena-Vázquez, N. et al.: Expansion of Rare and Harmful Lineages is Associated with Established Rheumatoid Arthritis. (2020) J. Clin. Med, | https://doi.org/10.3390/jcm9041044
Rothstein, M. et al.: The conversion of lysine to pipecolic acid in the rat. (1954) Journal of Biological Chemistry | https://doi.org/10.1016/S0021-9258(18)71173-3
Sell, D. et al.: 2-aminoadipic acid is a marker of protein carbonyl oxidation in the aging human skin: effects of diabetes, renal failure and sepsis. (2007) Biochem J, | https://doi.org/10.1042/BJ20061645
Toledo, J. et al.: Metabolic network failures in Alzheimer’s disease: A biochemical road map. (2017) Alzheimers Dement | https://doi.org/10.1016/j.jalz.2017.01.020
van Karnebeek, C. et al.: Lysine restricted diet for pyridoxine-dependent epilepsy: first evidence and future trials. (2012) Mol Genet Metab, | https://doi.org/10.1016/j.ymgme.2012.09.006
Wang, C. et al.: Knock-Out of DHTKD1 Alters Mitochondrial Respiration and Function, and May Represent a Novel Pathway in Cardiometabolic Disease Risk. (2021) Front Endocrinol | https://doi.org/10.3389/fendo.2021.710698
Wang, G. et al.: (2014) Plasma metabolite profiles of Alzheimer’s disease and mild cognitive impairment. (2014) J Proteome Res, | https://doi.org/10.1021/pr5000895
Wang, T. et al.: 2-Aminoadipic acid is a biomarker for diabetes risk. (2013) J Clin Invest, | https://doi.org/4309–4317
Xu, W.J. et al.: 2-Aminoadipic acid protects against obesity and diabetes. (2019) J Endocrinol | https://doi.org/10.1530/JOE-19-0157