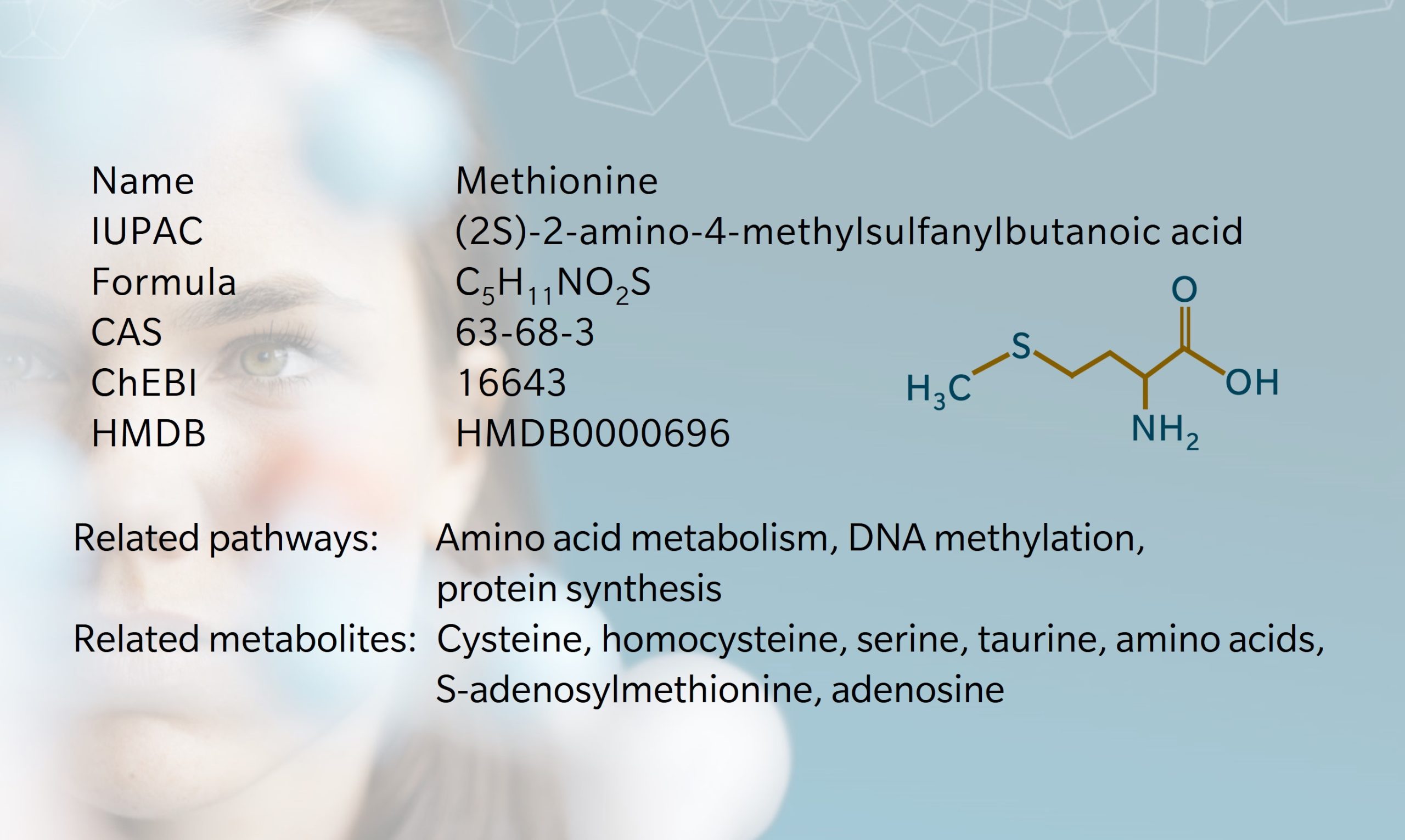
- History & Evolution
- Biosynthesis vs. dietary uptake
- Methionine and the microbiome
- Methionine and DNA methylation
- Methionine and cardiovascular disease
- Methionine and neurogenerative disorders
- Methionine and cancer
History & Evolution
1921: discovery (Mueller, J., 1921) | 1928: structure identified (Barger, G., 1928) | 1945-7: first synthesis (Livak, J. et al., 1945).
Methionine’s story begins just over a century ago, when it was discovered in casein by JH Mueller in 1921 (Mueller, J., 1921). In 1928, Barger and Coyne determined the chemical structure of what was until then known as γ-methylthiol-α-aminobutyric acid and suggested a more straightforward name: methionine (Barger, G., 1928). By the 1930s, methionine was recognized as an essential and limiting amino acid involved in protein synthesis, with subsequent research focusing on its vital role in human and animal diets (Rose, W., 1976). In the aftermath of World War II, protein shortages and widespread malnutrition prompted the hunt for a synthetic form of methionine (Livak, J. et al., 1945).
Now, over one million tons of methionine are produced each year from petroleum, with many applications including poultry feed, flavor enhancers, cosmetics, drugs and pesticides (Neubauer, C., 2021).
Methionine is found in humans, animals, plants and bacteria. In humans, methionine has been described as a “double-edged sword” (Navik, U. et al., 2021). It plays a crucial role in cellular, metabolic, epigenetic and genomic processes, influencing a broad range of physiological functions. It has been shown to support immune function, digestion, insulin resistance, to extend lifespan, and to reduce DNA damage and carcinogenesis (Martínez, Y. et al., 2017). However, excessive amounts can be toxic, with adverse effects including increased cholesterol and hyperhomocysteinemia (Navik, U. et al., 2021); (Garlick, P., 2006).
There is a theory that not only is this ancient metabolite essential for life, but – as a self-activating and sulfur containing amino acid – it may have triggered the development of life on Earth itself (Heinen, W., 1996).
Biosynthesis vs. dietary uptake
As an essential amino acid, methionine must be obtained via the diet and gut microbiota (Parkhitko, A. et al., 2019). Primary dietary sources of methionine include eggs, fish, meat, dairy and plant protein sources (Navik, U. et al., 2021) (Rajavel, E., 2020). In bacteria, methionine is synthesized from cysteine and aspartic acid. Plasma methionine concentration in healthy subjects ranges from 13 to 45 µM (Navik, U. et al., 2021).

The Quantitative Metabolomics Database (QMDB), cataloguing the concentrations of over 600 metabolites in healthy individuals reports average levels of 28 µM for women and 30 µM for men.
Methionine is one of four sulfur-containing amino acids, and, along with cysteine, one of two incorporated into proteins (Brosnan, T., 2006). Daily dietary requirements for methionine are usually taken together with cysteine, and the total sulfur amino acid requirement (TSAA) is 13-15 mg/kg/d for adults (Rajavel, E., 2020).
Methionine metabolism involves three key stages (Parkhitko, A. et al., 2019); (Lauinger, L., 2021):
– the methionine cycle,
– the transsulfuration/transmethylation pathway
– the methionine salvage cycle
In the methionine cycle, methionine and adenosine triphosphate (ATP) are converted into the methyl donor S-adenosylmethionine (SAM) by the enzyme methionine adenosyltransferase. After transferring the methyl group, SAM is converted to S-adenosylhomocysteine (SAH) and hydrolyzed into adenosine and homocysteine. This homocysteine can be remethylated to methionine by the methionine synthase enzyme (with vitamin B12 as a coenzyme), which completes the cycle, or diverted to the transssulfuration pathway to form cysteine (Rajavel, E., 2020); (Parkhitko, A. et al., 2019). In the salvage cycle, methionine is regenerated from decarboxylated SAM, following a series of enzymatic steps involving polyamine synthesis.
Methionine is a precursor to other molecules including antioxidants such as glutathione and cystathionine, and amino acids including cysteine, carnitine, taurine and creatine (Navik, U. et al., 2021).
Methionine and the microbiome
Gut microbiota play a major role in methionine metabolism, with around 20% of dietary methionine metabolized in the gut (Wu, X. et al., 2022); (Bauchart C. et al., 2009). In turn, methionine metabolism influences gut health, as evidenced by the interactions between methionine levels, microbial composition and disease states.
A multi-omics study comparing vegan and omnivore diets found that methionine positively correlates with Bacteroides, Blautia, Dorea, Lachnoclostridium and Fusicatenibacter, with all except Bacteroides being more abundant in omnivores (Prochazkova, M. et al., 2022). Another study found that restricting sulfur amino acids like methionine increases the abundance of Firmicutes, Clostridaceae and Turicibacteraceae, while decreasing Verrucomicrobia, compared to a low-calorie diet (Nichenametla, S. et al., 2021). These findings show how methionine intake influences the diversity and abundance of bacterial species in the gut.
Animal studies have shown that restricting dietary methionine, e.g. through a plant-based diet, may improve gut health. Methionine restriction in mice was found to increase short chain fatty acid (SCFA)-producing bacteria such as Bifidobacterium, Lactobacillus, Bacteroides, Roseburia, Coprococcus, and Ruminococcus, and increase inflammation-inhibiting bacteria like Oscillospira and Corynebacterium (Yang, Y. et al., 2019). In contrast, the high fat diet reduced SCFA production and induced gut dysbiosis.
Methionine appears to play a role in several inflammatory diseases associated with disrupted gut microbiota (Wu, X. et al., 2022). For example, a study in patients with inflammatory bowel disease (IBD) showed reduced levels of methionine, serine and sarcosine, along with fewer SCFA-producing gut bacteria (Borren, N. et al., 2021). Another study found changes in methionine and homoserine levels in patients with Parkinson’s disease (PD) (Hertel, J. et al., 2019). PD patients showed an increase in Akkermansia muciniphila, which is involved in sulfur metabolism. This contributed more than 70% of potential methionine production, suggesting a clear link between gut microbiota and the methionine cycle (Wu, X. et al., 2022).
Methionine and DNA methylation
Methionine is essential for DNA methylation, a key epigenetic mechanism affecting gene expression related to growth and development (Lauinger, L., 2021). In this process, DNA methyltransferases transfer a methyl group from SAM to the C-5 position of the pyrimidine ring of cytosine (Jin, B. et al., 2011). Changes in dietary methionine change the ratio of SAM to SAH (the “methylation index”), which influences methylation capacity. This can be measured using immunoassays to detect levels of SAM and SAH in blood and tissue (Hao, X. et al., 2016).
Dysregulation of DNA methylation is associated with some cancers (Bauchart C. et al., 2009). Studies suggest that methionine supplementation can affect DNA methylation, with both positive and negative effects depending on the gene region affected (Waterland, R., 2006).
More research is needed to fully understand methionine’s role in DNA methylation. Metabolomics offers a useful tool with which to understand epigenomics and DNA methylation in more detail, especially when combined with other omics. For example, a 2020 study combined metabolomics, proteomics and genomics to show that activated T cells use methionine to synthesize SAM, identifying methionine as a key nutritional factor in shaping T helper cell activity through histone methylation (Roy, D. et al., 2020). The findings suggest restricting dietary methionine could be a viable intervention in the treatment of autoimmune conditions such as multiple sclerosis.
Layering insights from different omics analyses in this way gives a fuller picture of the interactions between metabolites, proteins, genes and environmental factors that drive disease processes.
Methionine and cardiovascular disease
Too much methionine may increase the risk of cardiovascular disease (CVD). Methionine is involved in DNA synthesis, lipid regulation, oxidative defense and cellular regulation – processes all thought to play a role in CVD and other diseases (Aissa, A. et al., 2017). Impaired DNA methylation may also be a factor, as this increases circulating LDL cholesterol and lowers HDL cholesterol, both of which are risk factors for CVD (Calderón, A. et al., 2020); (Blachier, F. et al., 2020).

Another key mechanism is linked to methionine’s downstream metabolite homocysteine, which is known to be vasotoxic (Troen, A. et al., 2003). In excess, homocysteine can cause hyperhomocysteinemia, which is a well-established risk factor for CVD. A longitudinal population-based study found that a lower concentration of methionine, higher concentration of homocysteine and lower ratio of methionine to homocysteine were associated with an increased risk of CVD (Calderón, A. et al., 2020).
Excess methionine may also damage endothelial cells and increase plasma lipid levels, which both contribute to atherosclerosis. High levels of dietary methionine have been found to increase the risk of acute coronary events in middle-aged men (Virtanen, J. et al., 2006).
By contrast, methionine restriction appears to delay development of CVD and associated risk factors such as obesity (Zhang, Y. et al., 2022).
Methionine and neurogenerative disorders
Excess methionine is linked to neurodevelopmental disorders, like autism and schizophrenia, and neurodegenerative disorders such as Alzheimer’s disease (AD) and PD (Alachkar, A. et al., 2022); (Hertel, J. et al., 2019). A 2023 study involving animal and human AD phenotypes found that methionine intake is associated with mild cognitive impairment, and methionine restriction improves cognitive function (Xi, Y. et al., 2022).
More research is needed to understand the mechanisms, but again, methionine’s role in DNA methylation and homocysteine production may be relevant. A population-based study found links between methionine, homocysteine and dementia development, suggesting that a higher methionine to homocysteine ratio may reduce brain atrophy and lower the risk of dementia (Hooshmand, B. et al., 2019).
As noted above, a longitudinal metabolomics study investigated links between PD, sulfur metabolism and the gut microbiome (Hertel, J. et al., 2019). This showed differences between methionine and cysteine production via cystathionine in PD patients and healthy subjects. Using multiomics, the researchers were able to identify patterns in microbial-host sulfur metabolism that may contribute to PD severity.
Methionine and cancer
Methionine restriction is also an “exciting potential tool in the treatment of cancer” (Wanders, D. et al., 2020). Methionine inhibits cancer cell proliferation and growth in several types of cancer, without damaging healthy cells (in the presence of homocysteine). It also appears to improve efficacy of chemotherapy and radiation therapy in animal models. A 2020 review summarized evidence showing that methionine restriction could inhibit cancer development and/or progression in prostate cancer, breast cancer and colorectal cancer (Wanders, D. et al., 2020). Possible mechanisms vary by cancer type. It may be that methionine restriction inhibits polyamine biosynthesis, induces apoptosis, alters DNA methylation and glutathione formation, and reduces activity of thymidylate synthase, the enzyme that converts dUMP to dTMP.
References
Aissa, A. et al.: Methionine-supplemented diet affects the expression of cardiovascular disease-related genes and increases inflammatory cytokines in mice heart. (2017). J Toxicol Environ Health A | DOI: 10.1080/15287394.2017.1357366
Alachkar, A. et al.: L-methionine enhances neuroinflammation and impairs neurogenesis: implication for alzheimer’s disease. (2022). Journal of Neuroimmunology | DOI: 10.1016/j.jneuroim.2022.577843
Barger, G. et al.: The amino-acid methionine; constitution and synthesis. (1928). Biochem J. | DOI: 10.1042/bj0221417
Bauchart, C. et al.: Intestinal metabolism of sulfur amino acids. (2009). Research Reviews | DOI: 10.1017/S0954422409990138
Blachier, F. et al.: Sulfur-containing amino Acids and lipid metabolism. (2020). The Journal of Nutrition | DOI: 10.1093/jn/nxaa243
Borren, N. et al.: Alterations in fecal microbiomes and serum metabolomes of fatigued patients with quiescent inflammatory bowel diseases. (2021). Clin Gastroenterol Hepatol | DOI: 10.1016/j.cgh.2020.03.013
Brosnan, T. et al.: The sulfur-containing amino acids: an overview. (2006). J Nutr | DOI: 10.1093/jn/136.6.1636S
Calderón, A. et al.: Association of homocysteine, methionine, and mthfr 677c>t polymorphism with rate of cardiovascular multimorbidity development in older adults in sweden. (2020). JAMA Netw Open | DOI: 10.1001/jamanetworkopen.2020.5316
Garlick, P. et al.: Toxicity of methionine in humans. (2006). The Journal of Nutrition | DOI: 10.1093/jn/136.6.1722S
Hao, X. et al.: Immunoassay of S-adenosylmethionine and S-adenosylhomocysteine: the methylation index as a biomarker for disease and health status. (2016). BMC Res Notes | DOI: 10.1186/s13104-016-2296-8
Heinen, W. et al.: Organic sulfur compounds resulting from the interaction of iron sulfide, hydrogen sulfide and carbon dioxide in an anaerobic aqueous environment. (1996). Orig Life Evol Biosph | DOI: 10.1007/BF01809852
Hertel, J. et al.: Integrated analyses of microbiome and longitudinal metabolome data reveal microbial-host interactions on sulfur metabolism in parkinson’s disease. (2019). Cell Rep | DOI: 10.1016/j.celrep.2019.10.035
Hooshmand, B. et al.: Association of methionine to homocysteine status with brain magnetic resonance imaging measures and risk of dementia. (2019). JAMA Psychiatry | DOI: 10.1001/jamapsychiatry.2019.1694
Jin, B. et al.: DNA methylation. (2011). Genes Cancer | DOI: 10.1177/1947601910393957
Kaiser, P. et al.: Methionine dependence of cancer. (2020). Biomolecules | DOI: 10.3390/biom10040568
Lauinger, L. et al.: Sensing and signaling of methionine metabolism. (2021). Metabolites | DOI: 10.3390/metabo11020083
Livak, J. et al.: Synthesis of dl-methionine. (1945). J Am Chem Soc | DOI: 10.1021/ja01228a050
Martínez, Y. et al.: The role of methionine on metabolism, oxidative stress, and diseases. (2017). Amino Acids | DOI: 10.1007/s00726-017-2494-2
Mueller, J. et al.: Growth-determining substances in bacteriological culture media. (1921). Proceedings of the Society for Experimental Biology and Medicine | DOI: 10.3181/00379727-18-113
Navik, U. et al.: Methionine as a double-edged sword in health and disease: Current perspective and future challenges. (2021). Ageing Research Reviews | DOI: 10.1016/j.arr.2021.101500
Neubauer, C. et al.: A planetary health perspective on synthetic methionine. (2021). The Lancet Planetary Health | DOI: 10.1016/S2542-5196(21)00138-8
Nichenametla, S. et al.: Differential Effects of Sulfur Amino Acid-Restricted and Low-Calorie Diets on Gut Microbiome Profile and Bile Acid Composition in Male C57BL6/J Mice. (2021). The Journals of Gerontology: Series A | DOI: 10.1093/gerona/glaa270
Parkhitko, A. et al.: Methionine metabolism and methyltransferases in the regulation of aging and lifespan extension across species. (2019). Aging Cell | DOI: 10.1111/acel.13034
Prochazkova, M. et al.: Vegan diet Is associated with favorable effects on the metabolic performance of intestinal microbiota: A cross-sectional multi-omics study. Front. (2022). Nutr | DOI: 10.3389/fnut.2021.783302
Rajavel, E. et al.: Methionine Nutrition and Metabolism: Insights from Animal Studies to Inform Human Nutrition. (2020). The Journal of Nutrition | DOI: 10.1093/jn/nxaa155
Rose, W. et al.: Amino Acid Requirements of Man. (1976). Nutrition Reviews | DOI: 10.1111/j.1753-4887.1976.tb05679.x
Roy, D. et al.: Methionine metabolism shapes t helper cell responses through regulation of epigenetic reprogramming. (2020). Cell Metab | DOI: 10.1016/j.cmet.2020.01.006
Troen, A. et al.: The atherogenic effect of excess methionine intake. (2003). PNAS | DOI: 10.1073/pnas.2436385100
Virtanen, J. et al.: High dietary methionine intake increases the risk of acute coronary events in middle-aged men. (2006). Nutr Metab Cardiovasc Dis | DOI: 10.1016/j.numecd.2005.05.005
Wanders, D. et al.: Methionine restriction and cancer biology. (2020). Nutrients | DOI: 10.3390/nu12030684
Waterland, R. et al.: Assessing the Effects of High Methionine Intake on DNA Methylation. (2006). The Journal of Nutrition | DOI: 10.1093/jn/136.6.1706S
Wu, X. et al.: Gut microbiota contributes to the methionine metabolism in host. (2022). Front Microbiol | DOI: 10.3389/fmicb.2022.1065668
Xi, Y. et al.: Effects of methionine intake on cognitive function in mild cognitive impairment patients and APP/PS1 Alzheimer’s Disease model mice: Role of the cystathionine-β-synthase/H2S pathway. (2022). Redox Biol | DOI: 10.1016/j.redox.2022.102595
Yang, Y. et al.: Dietary methionine restriction improves the gut microbiota and reduces intestinal permeability and inflammation in high-fat-fed mice. (2019). Food and Function | DOI: 10.1039/C9FO00766K
Zhang, Y. et al.: Methionine restriction – Association with redox homeostasis and implications on aging and diseases. (2022). Redox Biology | DOI: 10.1016/j.redox.2022.102464