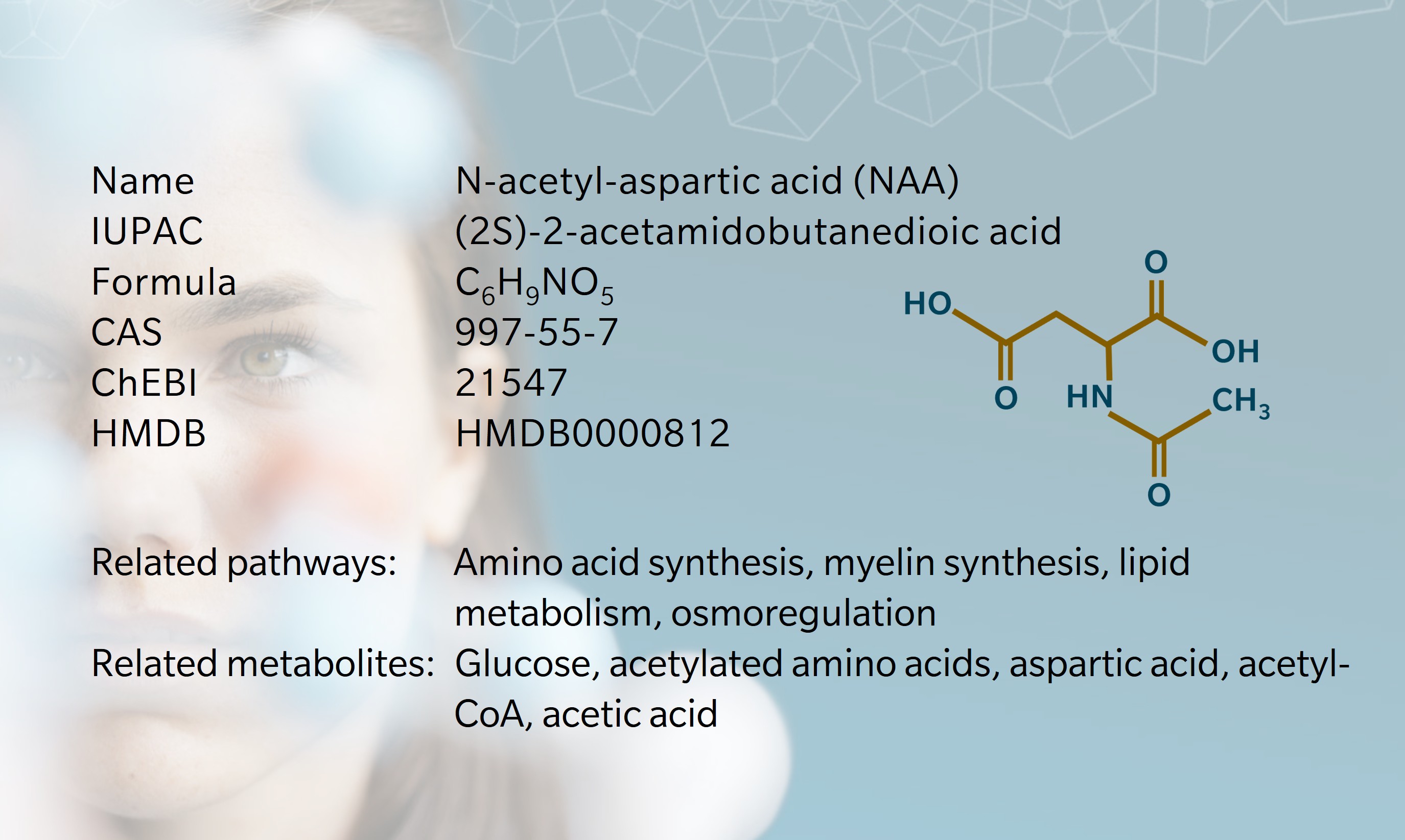History & Evolution
1956: discovery (Tallan et al. 1956) | 1959: first synthesis (Goldstein 1959)
N-acetyl-aspartic acid (NAA), or N-acetylaspartate, is one of the most abundant metabolites in the mammalian central nervous system (CNS) (Long et al. 2013). Despite extensive research since its discovery in 1956 (Tallan et al. 1956), it remains something of a biochemical enigma (Moffett et al. 2007). It is known to be involved in osmoregulation, myelin synthesis, neuronal metabolism and acetate storage for lipid metabolism, and disruption to the NAA pathway has demonstrable physiological effects (Moffett et al. 2007). However, researchers continue to debate its primary function and mechanisms of action (Bogner-Strauss 2017).
An important insight came in 1959, when Goldstein successfully synthesized NAA using acetone powders derived from rat brain tissue (Goldstein 1959). This showed that NAA is formed in neuronal mitochondria from aspartic acid and acetyl-coenzyme A (CoA).
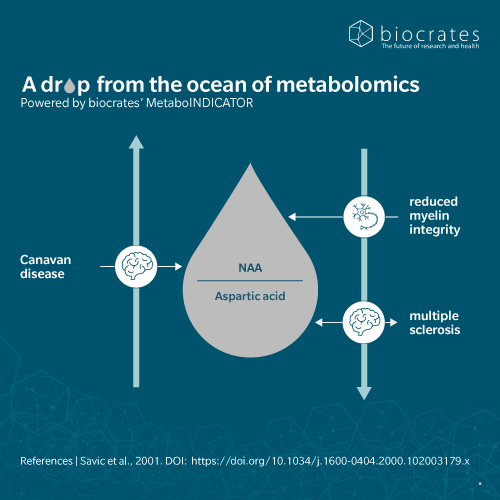
Two further notable findings brought NAA to the attention of the neurological community (Moffett et al. 2007). First, its strong signal in magnetic resonance spectroscopy (MRS) made it a reliable marker in brain imaging. Second, researchers discovered that a deficiency of the enzyme aspartoacylase leads to NAA accumulation in the brain, causing the rare genetic disorder known as Canavan disease. These breakthroughs confirmed NAA’s role as a diagnostic tool and key player in neurological disease.
There are hints that NAA is also involved in broader cognitive processes: NAA has been nicknamed the “creativity chemical,” (Geddes 2009) and associates with memory and educational attainment (Glodzik et al. 2012).
Biosynthesis vs. dietary uptake
NAA is primarily synthesized endogenously. N-acetyl amino acids are synthesized through the breakdown of N-acetyl proteins or direct acetylation of amino acids by specific enzymes (Ramunaidu et al. 2023). In the case of NAA, N-acetylaspartate synthetase (encoded by NAT8L, a gene highly expressed in brain and connective tissue) catalyzes a reaction between acetyl-CoA and aspartic acid to produce NAA in neuronal mitochondria. NAA is transported to oligodendrocytes where it is hydrolyzed by aspartoacylase, producing L-aspartate and acetate (Moffett et al. 2007). Acetate is then incorporated into acetyl-CoA, which is essential for energy production, lipid synthesis and protein acetylation in the brain.
NAA synthesis also occurs outside the CNS (Bogner-Strauss 2017). Research shows that brown adipose tissue (BAT) expresses significant levels of NAT8L, allowing it to synthesize NAA (Huber et al. 2018). While NAA itself is not obtained directly from the diet, dietary intake can influence the availability of its precursors, such as glucose and aspartate, which are abundant in BAT. A study of mice fed high-fat and high-glucose diets found that both conditions increased NAA pathway activity in brown adipocytes compared to standard diet, in turn affecting energy and lipid metabolism (Huber et al. 2018).
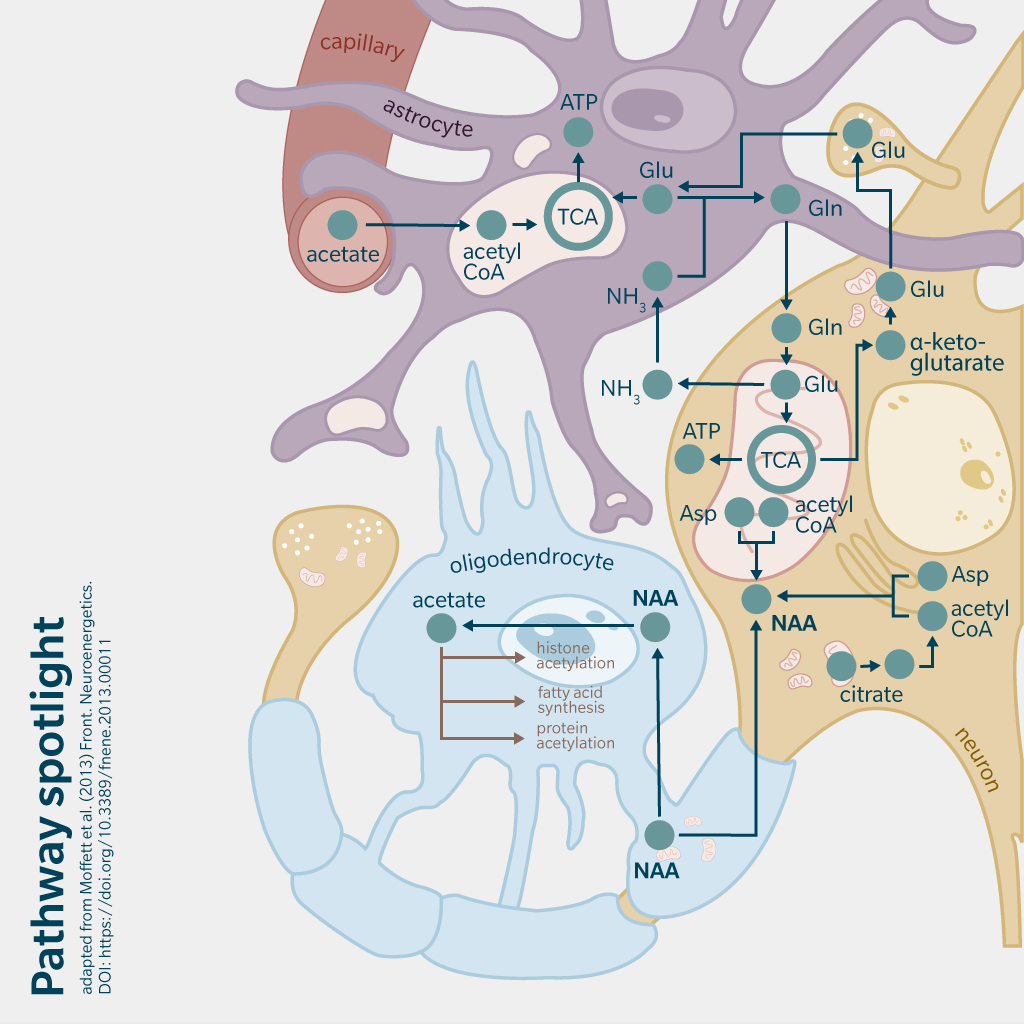
NAA and the microbiome
While gut microbiota do not produce or consume NAA in significant amounts, microbial composition has been shown to influence cerebral metabolite levels, including NAA (Matsumoto et al. 2013).
Research into maternal postpartum depression (PPD) and infant neurodevelopment highlights this connection (Zhou et al. 2024). Infants born to mothers with PPD symptoms exhibited increased Veillonella and reduced Bifidobacterium, as well as lower levels of NAA and aspartate in their gut metabolome. These changes were associated with poorer neurodevelopmental scores at six months, suggesting that maternal mental health and microbiome alterations can indirectly affect brain metabolites and neurodevelopment.
Similarly, an animal model showed that fecal Ruminococcus and Butyricimonas predicted NAA levels in the brain, suggesting a potential mechanism for microbiota-brain communication in neurological conditions such as autism spectrum disorder (Mudd et al. 2017).
NAA and neurology
NAA makes up around 0.1% of the brain’s net weight (Inglese et al. 2008). Its abundance makes it a useful marker of neuronal health, measured via magnetic resonance spectroscopy (MRS). Altered levels of NAA have been observed in several brain diseases and disorders (Moffett et al. 2007).
The discovery of the connection between NAA and Canavan disease marked a major step forward in understanding the role of NAA in neurological disease (Moffett et al. 2007). In Canavan disease, a mutation in the ASPA gene impairs NAA breakdown, causing an accumulation of NAA and progressive leukodystrophy in infants. Clearly, excess NAA can be harmful. At the same time, reduced NAA levels have been linked to neuronal loss and degeneration in other conditions, for example:
Alzheimer’s disease
Patients with Alzheimer’s disease have reduced NAA levels in specific brain regions, correlating with neuronal loss and cognitive decline (Schuff et al. 2006) (Moffett et al. 2007).
Multiple sclerosis
NAA deficiency is associated with neurodegeneration and is considered a hallmark of the disease, demonstrated using MRS and HPLC analysis. A 2023 study found that in patients with MS, oxidized NAT8L mRNA limits NAT8L production and reduces NAA levels, establishing a molecular link between NAA, mRNA oxidization and MS pathogenesis (Kharel et al. 2023).
Traumatic brain injury
A review of 20 publications with metabolomics investigations into severe traumatic brain injury found that NAA levels drop after injury, reflecting neuronal damage (Fedoruk et al. 2023).
Psychiatric disorders
A 2018 review of metabolomics studies for psychosis identified NAA as a potential biomarker for psychosis (Li et al. 2018). In patients with chronic major depressive disorder, NAA concentrations were significantly reduced in several brain regions compared to healthy controls. Antidepressant treatment appeared to reduce NAA alterations in the frontal lobe.
NAA and oncology
Recent research points to the involvement of the NAA pathway in cancer biology. Elevated NAA levels have been observed in several cancer types, including non-small-cell lung cancer, breast cancer, ovarian cancer, and prostate cancer (Krause et al. 2024). A 2016 study using untargeted metabolic profiling found that NAA levels in high-grade serous ovarian cancer (HGSOC) tissue were more than 28 times higher than in normal ovarian tissue, higher than any other amino acid metabolite (Zand et al. 2016). Another multi-omics study demonstrated the accumulation of NAA in murine models of castration-resistant prostate cancer (Salji et al. 2022).
One possible mechanism is that NAA impacts key cellular processes such as histone acetylation and signaling pathways that promote tumor growth and survival. Additionally, reduced expression of NAT8L, which drives NAA synthesis, is linked to reduced cancer proliferation, further linking NAA to tumor growth (Krause et al. 2024).
These findings suggest that NAA metabolism holds promise as a biomarker and therapeutic target oncology.
NAA and 5P medicine
Metabolomics is helping researchers understand more about the role of NAA in disease monitoring and personalized treatment planning, in both chronic and acute disease, and beyond neurology and oncology. For example, a 2022 metabolomics study identified NAA as a potential biomarker of juvenile idiopathic arthritis and cardiovascular disease risk (Lewis et al. 2022).
Another study used MRS to evaluate the impact of disease severity on brain metabolites in cases of COVID-19 (Ostojic et al. 2024). NAA levels were significantly lower in more severe cases, suggesting neuronal dysfunction.
Investigations like these reveal more about NAA pathways, offering insights for predictive diagnostics, personalized treatment and population health strategies. With continued research, NAA may soon cease to be the enigma it once was.
References
Bogner-Strauss, J.: N-Acetylaspartate Metabolism Outside the Brain: Lipogenesis, Histone Acetylation, and Cancer (2017) Frontiers in Endocrinology, 8, 240. | DOI: https://doi.org/10.3389/fendo.2017.00240.
Fedoruk, R. et al.: Metabolomics in severe traumatic brain injury: a scoping review (2023) BMC Neurosci., 24, 52. | DOI: https://doi.org/10.1186/s12868-023-00824-1.
Geddes, L. (2009, May 13). Creativity chemical favours the smart. Retrieved from New Scientist: https://www.newscientist.com/article/mg20227084-300-creativity-chemical-favours-the-smart/
Glodzik, L. et al.: The whole-brain N-acetylaspartate correlates with education in normal adults (2012) Psychiatry Research: Neuroimaging, 204(1), 49-54. | DOI: https://doi.org/10.1016/j.pscychresns.2012.04.013.
Goldstein, F.: Biosynthesis of N-Acetyl-l-aspartic Acid. Journal of Biological Chemistry (1959) 234(10), 2702-2706. | DOI: https://doi.org/10.1016/S0021-9258(18)69763-7.
Huber, K. et al.: N-acetylaspartate pathway is nutrient responsive and coordinates lipid and energy metabolism in brown adipocytes (2018) Biochim Biophys Acta Mol Cell Res., 1866(3), 337–348. | DOI: https://doi.org/10.1016/j.bbamcr.2018.08.017.
Inglese, M. et al.: Global Average Gray and White Matter N-acetylaspartate Concentration in the Human Brain (2008) Neuroimage., 41(2), 270–276. | DOI: https://doi.org/10.1016/j.neuroimage.2008.02.034.
Kharel, P. et al.: NAT8L mRNA oxidation is linked to neurodegeneration in multiple sclerosis (2023) Cell Chemical Biology, 30(3), 308 – 320.e5. | DOI: https://doi.org/10.1016/j.chembiol.2023.02.007.
Krause, N. and Wegner, A.: N-acetyl-aspartate metabolism at the interface of cancer, immunity, and neurodegeneration (2024) Current Opinion in Biotechnology, 85, 103051. | DOI: https://doi.org/10.1016/j.copbio.2023.103051.
Lewis, K. et al.: Serine, N-acetylaspartate differentiate adolescents with juvenile idiopathic arthritis compared with healthy controls: a metabolomics cross-sectional study (2022) Pediatric Rheumatology, 20(12). | DOI: https://doi.org/10.1186/s12969-022-00672-z.
Li, C. et al.: Metabolomics in patients with psychosis: A systematic review (2018) American Journal of Medical Genetics: Neuropsychiatric Genetics, 177(6), 580-588. | DOI: https://doi.org/10.1002/ajmg.b.32662.
Long. P. et al.: N-Acetylaspartate (NAA) and N-Acetylaspartylglutamate (NAAG) Promote Growth and Inhibit Differentiation of Glioma Stem-like Cells (2013) Journal of Biological Chemistry, 288(36), 26188-26200. | DOI: https://doi.org/10.1074/jbc.M113.487553.
Matsumoto, M. et al.: Cerebral low-molecular metabolites influenced by intestinal microbiota: a pilot study (2013) Front. Syst. Neurosci., 7. | DOI: https://doi.org/10.3389/fnsys.2013.00009.
Moffett, J. et al.: N-Acetylaspartate in the CNS: From Neurodiagnostics to Neurobiology (2007) Prog Neurobiol., 81(2), 89–131. | DOI: https://doi.org/10.1016/j.pneurobio.2006.12.003.
Mudd, A. et al.: Serum cortisol mediates the relationship between fecal
Ruminococcus and brain N-acetylaspartate in the young pig (2017) Gut Microbes, 8(6), 589–600. | DOI: https://doi.org/10.1080/19490976.2017.1353849.
Ostojic, J. et al.: Decreased Cerebral Creatine and N-Acetyl Aspartate Concentrations after Severe COVID-19 Infection: A Magnetic Resonance Spectroscopy Study (2024) J Clin Med., 13(14), 4128. | DOI: https://doi.org/10.3390/jcm13144128.
Ramunaidu, A. et al.: Characterization of isomeric acetyl amino acids and di-acetyl amino acids by LC/MS/MS (2023) Journal of Mass Spectrometry, 58(12), e4982. | DOI: https://doi.org/10.1002/jms.4982.
Salji, M. et al.: Multi-omics & pathway analysis identify potential roles for tumor N-acetyl aspartate accumulation in murine models of castration-resistant prostate cancer (2022) iScience, 25(4), 104056. | DOI: https://doi.org/10.1016/j.isci.2022.104056.
Schuff, N. et al.: N-Acetylaspartate As A Marker Of Neuronal Injury In Neurodegenerative Disease (2006) Adv Exp Med Biol., 576, 241–363. | DOI: https://doi.org/10.1007/0-387-30172-0_17.
Tallan, H. Moore, S. and Stein, W.: N-Acetyl-L-Aspartic Acid In Brain (1956) Journal of Biological Chemistry, 219(1), 257-264. | DOI: https://doi.org/10.1016/S0021-9258(18)65789-8.
Zand, B. et al.: Role of Increased n-acetylaspartate Levels in Cancer (2016) JNCI J Natl Cancer Inst, 108(6), djv426. | DOI: https://doi.org/10.1093/jnci/djv426.
Zhou L. et al.: Association of maternal postpartum depression symptoms with infant neurodevelopment and gut microbiota (2024) Front Psychiatry., 15, 1385229. | DOI: https://doi.org/10.3389/fpsyt.2024.1385229.