History & Evolution
2007: atmospheric fingerprinting of pinene enantiomers | 2010: pinenes considered as renewable alternative to fuel | 2014: engineering of pinene-producing E. coli.
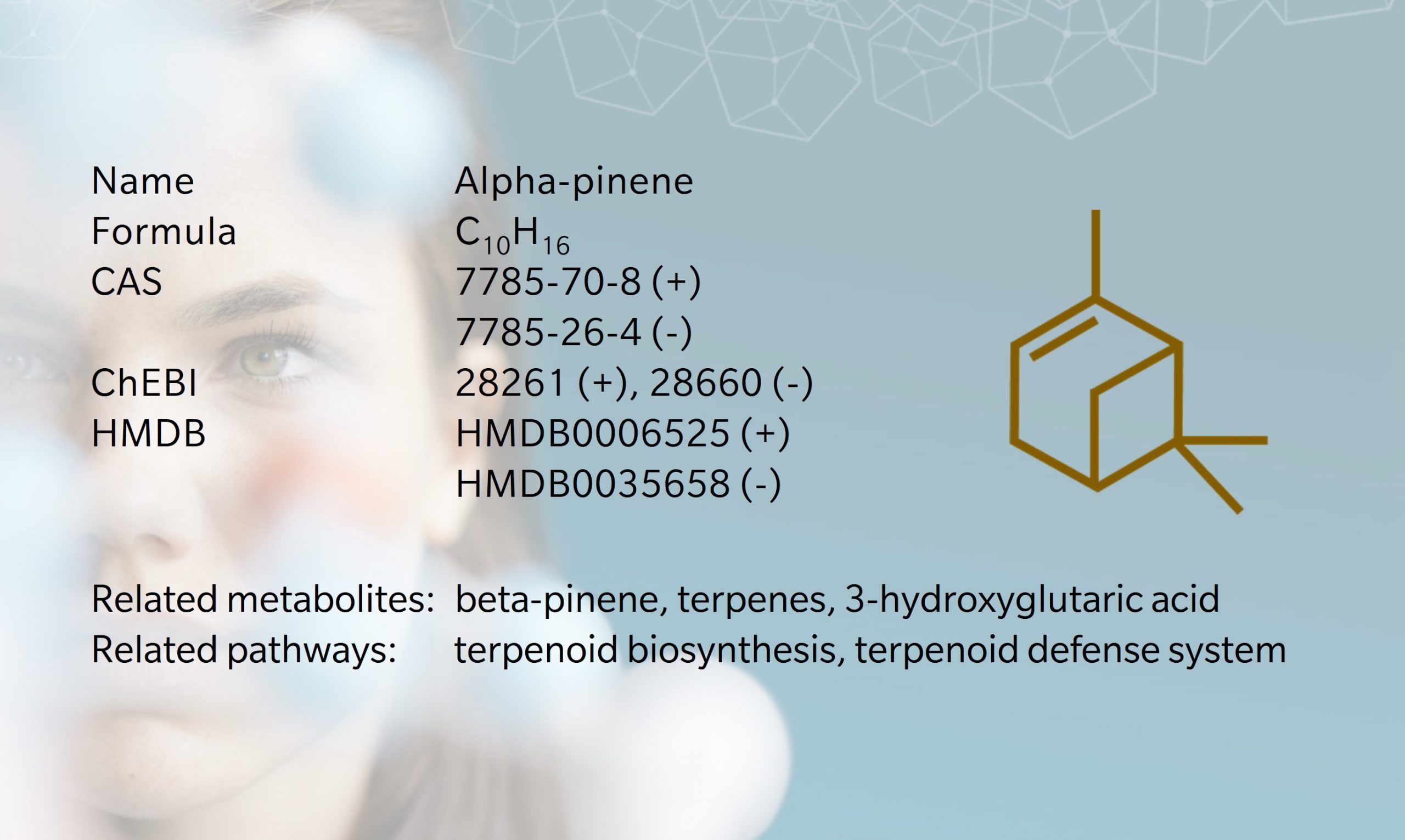
As one of the metabolites responsible for the smell of conifers (Schreiner et al. 2018), alpha-pinene is a fitting choice for our festive Metabolite of the month. This chiral molecule has two enantiomers that are found in varying proportions in different plants, as does its isomer, beta-pinene (Stephanou 2007).
Pinenes are unsaturated monoterpenes characterized by two rings fused to each other, making them quite reactive when released in the atmosphere. For instance, photooxidation of pinenes generates the metabolite 3-hydroxyglutaric acid (Claeys et al. 2007). Despite the clue in their name, their synthesis is not limited to pine trees and occurs in many plant species and some microorganisms.
A 2010 study identified beta-pinene as a potential renewable energy source, with properties comparable to those of jet fuel (Harvey et al. 2010). To enable industrial production of pinenes, bacterial strains were engineered to synthesize pinene by expressing pinene synthase and geranyl diphosphate synthase genes (Sarria et al. 2014). Beta-pinene was also used as a substrate to synthesize a high molecular weight polymer of interest for optoelectronics (Satoh et al. 2014; Winnacker 2018).
Pinenes also serve many endogenous functions in plants, with some interesting applications for human health, as discussed below.
Biosynthesis vs. dietary uptake
In plants, pinene biosynthesis starts with the activation of isoprene units that exist in two forms that react together to form geranyl pyrophosphate (GPP). GPP is transformed to its isomer linaloyl pyrophosphate, which undergoes cyclization and a nucleophilic attack, leading to a pinane cation intermediate. Alpha-pinenes are synthesized by methylene proton elimination while beta-pinenes arise from methyl proton elimination (Nyamwihura et al. 2022). Pinenes also serve as intermediates in the synthesis of other plant metabolites such as carvone (a common component of essential oils used in the food industry).
In human males, ingestion of oils containing pinenes resulted in absorption of alpha- and beta-pinene, with both metabolites detectable in plasma after 24 hours (Papada et al. 2020). A 2012 study showed that alpha-pinene and other terpenes were detectable in the plasma and milk of goats after ingestion, suggesting that these metabolites may be useful in tracing the consumption of certain foods by farm animals (Poulopoulou et al. 2012). However, terpene levels in cheese made from these goats’ milk were found to vary, which may limit their use as a feed tracer in processed milk products.
Alpha-pinene functions in plants
Pinenes appear to serve several functions in the plants that synthesize them. Studies have shown that pinenes help protect plants from pests, such as the red turpentine beetle, (Dendroctonus valens) (Xu et al. 2016) and the white pine weevil (Pissodes strobi) (McKay et al. 2003). The study by McKay et al. describes how traumatic resin ducts form in the Sitka spruce (Picea sitchensis) upon attack by pests that activate the tree’s terpenoid defense systems.
Pinenes also play a role in plant-to-plant communication. The release of pinenes into the atmosphere is light- and temperature-dependent (Stephanou 2007), and headspace exposure to these pinenes can cause strong reactions. For example, when Arabidopsis thaliana is exposed to a mixture of alpha and beta-pinenes, this triggers the plant’s defense responses, accumulation of reactive oxygen species, and changes in gene expression that are consistent with systemic acquired resistance (Riedlmeier et al. 2017). Thus, pinenes and other monoterpenes are infochemicals, supporting plant-to-plant signaling and propagating defense signals between neighboring plants.
Lastly, pinenes influence the growth of their host plant. In Cicer arietinum, alpha-pinene increases solute leakage from roots and increases the levels of proline, malondialdehyde and hydrogen peroxide, inhibiting radicle growth (Singh et al. 2006). Beta-pinene has been found to inhibit germination in several weed species (Chowhan et al. 2013).
Alpha-pinene activity in animals
Pinenes are not only relevant to plant biology.They have been found to have several properties that are of interest in human health and disease. Pinenes have been studied for their antimicrobial properties (Da Rivas et al. 2012). They have also been described as having anticoagulant, antitumor, antimalarial, antioxidant, anti-inflammatory, and analgesic (Salehi et al. 2019). Some of these effects were uncovered through the study of active ingredients in the plants used in traditional Chinese medicine. For example, a 2011 study showed that alpha-pinene derivatives isolated from Angelica sinensis inhibited platelet aggregation and exhibited weak antithrombin activity (Yang et al. 2011).
Pinenes are also studied for their effects on cancer and cancer therapies. Both alpha- and beta-pinenes have shown synergistic antitumor effects with paclitaxel in the treatment of non-small-cell lung carcinoma (Zhang et al. 2015). Interestingly, in a murine model, exposure to a fragrant environment rich in alpha-pinene prior to and after tumor implantation resulted in 40% smaller tumors, increased plasma leptin levels, and neurological and immune differences compared to mice who were not exposed to alpha-pinene (Kusuhara et al. 2019).
In various models, pinenes were also shown to increase the skin penetration of drugs (Almirall et al. 1996), provide anti-inflammatory effects via inhibition of mitogen-activated protein kinases (MAPK) and nuclear factor-kappa B (NF-κB) in macrophages (Kim et al. 2015), influence vascular tone (Jin et al. 2023), and have a protective effect on the gastrointestinal tract (Marcelo de Almeida et al. 2015).
Alpha-pinene in the atmosphere
Researchers collect samples of the air over forests to identify the enantiomeric composition of the volatile compounds emitted by trees. A 2007 comparison of the ambient air over tropical (South America) and boreal (Northern Europe) forests showed that (-) alpha-pinene and (+) beta-pinene were predominant in the tropical forest while (+) alpha-pinene and (-) beta-pinene were predominant in the boreal forest (Williams et al. 2007). These results indicate a fingerprint-like profile of pinene enantiomers between different ecosystems and raise questions about the effects of such enantiomeric mixtures in the atmosphere.
To continue learning about seasonal metabolites, read our article on cinnamaldehyde.
References
Almirall M. et al.: Effect of d-limonene, alpha-pinene and cineole on in vitro transdermal human skin penetration of chlorpromazine and haloperidol. (1996) In Arzneimittel-Forschung | Available online at https://pubmed.ncbi.nlm.nih.gov/8842336/
Chowhan, N. et al.: β-Pinene inhibited germination and early growth involves membrane peroxidation. (2013) In Protoplasma http://doi.org/10.1007/s00709-012-0446-y
Claeys M. et al.: Hydroxydicarboxylic acids: markers for secondary organic aerosol from the photooxidation of alpha-pinene. (2007) Environmental Science & Technology | http://doi.org/10.1021/es0620181
Da Rivas S. et al.: Biological activities of α-pinene and β-pinene enantiomers. (2012) In Molecules 17 | http://doi.org/10.3390/molecules17066305
Harvey B. et al.: High-Density Renewable Fuels Based on the Selective Dimerization of Pinenes. (2010) Energy Fuels 24 | http://doi.org/10.1021/ef900799c
Jin L. et. al.: Endothelial-dependent relaxation of α-pinene and two metabolites, myrtenol and verbenol, in isolated murine blood vessels. In American journal of physiology. (2023) Heart and circulatory physiology 325 | http://doi.org/10.1152/ajpheart.00380.2023
Kim D. et al.: Alpha-Pinene Exhibits Anti-Inflammatory Activity Through the Suppression of MAPKs and the NF-κB Pathway in Mouse Peritoneal Macrophages. (2015) The American journal of Chinese medicine 43 | http://doi.org/10.1142/S0192415X15500457
Kusuhara M. et al.: (2019): A Fragrant Environment Containing α-Pinene Suppresses Tumor Growth in Mice by Modulating the Hypothalamus/Sympathetic Nerve/Leptin Axis and Immune System.(2019) Integrative cancer therapies 18 | http://doi.org/10.1177/1534735419845139
Marcelo de Almeida P. et al.: Gastroprotective effect of alpha-pinene and its correlation with antiulcerogenic activity of essential oils obtained from Hyptis species. (2015) In Pharmacognosy Magazine | Available online at https://phcog.com/article/view/2015/11/41/123-130
McKay S. et al.: Insect attack and wounding induce traumatic resin duct development and gene expression of (-)-pinene synthase in Sitka spruce. (2003) Plant physiology | http://doi.org/10.1104/pp.103.022723
Nyamwihura R. et al.: The pinene scaffold: its occurrence, chemistry, synthetic utility, and pharmacological importance. (2022) RSC advances | http://doi.org/10.1039/d2ra00423b
Papada E. et al.: An Absorption and Plasma Kinetics Study of Monoterpenes Present in Mastiha Oil Humans. (2020) Foods 9 | http://doi.org/10.3390/foods9081019
Poulopoulou I. et.al.: Transfer of orally administered terpenes in goat milk and cheese. (2012) Asian-Australas J Anim Sci 25 | http://doi.org/10.5713/ajas.2012.12165
Riedlmeier M. et al.: Monoterpenes Support Systemic Acquired Resistance within and between Plants. (2017) The Plant cell | http://doi.org/10.1105/tpc.16.00898
Salehi B. et al.: Therapeutic Potential of α- and β-Pinene: A Miracle Gift of Nature. (2019) Biomolecules | http://doi.org/10.3390/biom9110738
Sarria, S. et al.: Microbial synthesis of pinene. (2014) ACS Synthetic Biology | http://doi.org/10.1021/sb4001382
Satoh K. et al.: Sustainable cycloolefin polymer from pine tree oil for optoelectronics material: living cationic polymerization of β-pinene and catalytic hydrogenation of high-molecular-weight hydrogenated poly(β-pinene). (2014) Polym. Chem. | http://doi.org/10.1039/C3PY01320K
Schreiner L. et al.: Resolving the smell of wood – identification of odour-active compounds in Scots pine (Pinus sylvestris L.). (2018) Sci Rep 8 (1), p. 8294. | http://doi.org/10.1038/s41598-018-26626-8
Singh H. et al.: alpha-Pinene inhibits growth and induces oxidative stress in roots. (2018) Annals of botany | http://doi.org/10.1093/aob/mcl213
Stephanou E.G.: Atmospheric chemistry: a forest air of chirality. (2007) Nature | http://doi.org/10.1038/446991a
Williams J. et.al.: Mirror image hydrocarbons from Tropical and Boreal forests. (2007) Atmos. Chem. Phys.| http://doi.org/10.5194/acp-7-973-2007
Winnacker M.: Pinenes: Abundant and Renewable Building Blocks for a Variety of Sustainable Polymers. (2018) Angewandte Chemie (International ed. in English) | http://doi.org/10.1002/anie.201804009
Xu, L. et al.: Pine Defensive Monoterpene α-Pinene Influences the Feeding Behavior of Dendroctonus valens and Its Gut Bacterial Community Structure. (2016) International Journal of Molecular Sciences | http://doi.org/10.3390/ijms17111734
Yang N. et al.: Two new α-pinene derivatives from Angelica sinensis and their anticoagulative activities. (2011) Fitoterapia | http://doi.org/10.1016/j.fitote.2011.02.007
Zhang Z. et al.: Synergistic antitumor effect of α-pinene and β-pinene with paclitaxel against non-small-cell lung carcinoma (NSCLC). (2015) Drug research | http://doi.org/10.1055/s-0034-1377025