Metabolomics in preclinical and clinical research


Metabolomics in preclinical and clinical research
Pharmacometabolomics
Lack of translatability from preclinical to clinical stages, toxicities and non-response remain prevalent in drug development, leading to high attrition rates across indications. Metabolomics has proven to be a versatile tool across the pharmaceutical R&D cycle, improving the understanding of how new therapeutic agents act, ultimately providing insights for personalized therapies.
Metabolomics increases success rates at all stages of pharmaceutical development
Only around one in 5,000 compounds tested in the discovery stage are approved for routine use.
Even then, further limitations arise in real-world settings
- Non-response is common, with more than 1 in 3 patients experiencing no clinical benefit
- Drugs may become ineffective due to acquired resistance mechanisms and/or progression of disease
- There is a lack of actionable biomarkers to determine the best course of therapy
See metabolomics in action across the research cycle
A quantitative metabolomics biomarker strategy increases the success probability of your compound
Discovery
- Mode of action
- Compound prioritization
Preclinical
research
- Response biomarker
- Dosage | mode of delivery
- Molecular toxicology
Clinical
development
- Dose verification
- Safety biomarker
- Resistance or relapse
Approval |
Market
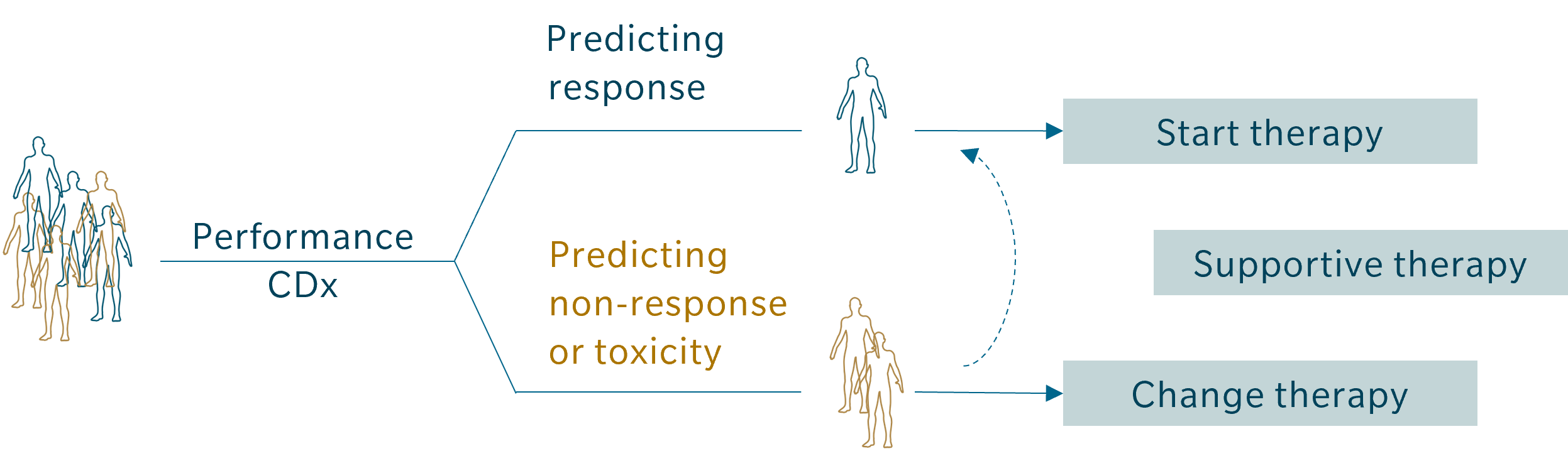
- Patient stratification
- Prediction of response
- Performance CDx
Discovery
Preclinical
research
Clinical
development
Approval |
Market
In vitro studies | Cell lines
- Leverage the fact that metabolism is highly conserved across species
- Replicate findings from previous academic research with standardized quantification
Ease your choice
- Test more parameters to prioritize the most useful
- Understand relevant metabolic pathways using thousands of metabolites and over 400 metabolism indicators
Case study
Advancing cancer treatment by targeting dysregulated metabolism – A roadmap
In this webinar, Dr. Anna Halama gives an overview of the application of metabolomics in the mechanistic study of cancer in cell cultures and xenografts.

Anna Halama, Ph.D.
Weill Cornell Medicine | Qatar
Open in YouTube
Discovery
Preclinical
research
Clinical
development
Approval |
Market
Translate insights from "mouse to man"
- Toxicological parameters are reflected in metabolic alterations
- Mode of action is reflected in metabolic alterations
- Metabolism is highly conserved across species
Improve translation with preferred technology
- Metabolomics is highly sensitive
- Low sample volume allows sequential sampling to reduce animal demand
- Understand relevant metabolic pathways using over 400 metabolism indicators
Case study – PI3K inhibition metabolomic biomarkers
22 of 26 dose-dependent metabolic mouse biomarkers successfully translate into clinical research phase I
(ICR London)
Read the full paper by Ang et al., 2016
Request more case studies – Preclinical and translational research
Talk to an expert
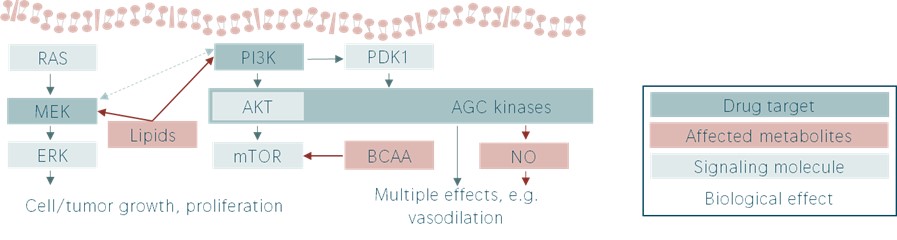
Metabolic effects of different cancer drugs in the same signalling network.
(adapted from work by Florence Raynaud)
Discovery
Preclinical
research
Clinical
development
Approval |
Market
Increase clinical potential
- Identify likely responders
- Get actionable insights for patient care and therapy optimization
- Keep patient safety in check over the whole trial period
Scalable and reproducible solution
- Short turn-around times
- Closely observe patients through scalable turn-around times
- Measure safety- and response biomarkers in different laboratories around the world
Commentary – Adverse effects in immunotherapy
There is growing evidence that metabolomics can provide useful biomarkers for patient outcomes in immunotherapy. Stefan Ledinger, Director of Business Development at biocrates, summarizes views on the role of metabolism in regulating immune responses and perturbations in immunometabolic pathways.
Discovery
Preclinical
research
Clinical
development
Approval |
Market
Optimize treatment
- Tailor treatment to patients’ individual biochemical response
- Improve treatment outcomes and achieve higher patient satisfaction
- Deliver results from precision medicine to precision health
Implement and scale with ease
- Laboratory onboarding takes less than a week
- Fully quality-controlled kit solution
- Globally certified partner network to scale throughput to match demand
Case study
Metabolomics leading the way from stratification to precision medicine
Metabolomics was used to understand immunotherapy response in urological cancers, revealing unconventional supplementation options to make drug treatment more effective.
“I am a biotech and pharma scientist, and I worked on metabolomics platform development and application since year 2000. biocrates is one of the few companies providing a standardized and validated large-scale metabolomics analysis that generates consistently reproducible data. I came to biocrates for its in-depth coverage of metabolism pathways and highly accurate quantitation via a targeted approach.
Most recently, to investigate the mode of action (MoA) of a research drug candidate, I used the Quant 500 XL platform that quantifies small molecules of critical metabolism pathways and a large number of lipids. With this comprehensive panel and accurate quantitation, I was able to obtain great insights into metabolic processes and pathways perturbed by our molecule. biocrates’ team of experts is highly professional, the analytical team is meticulous and knowledgeable about their workflow from sample prep to data analysis. The data analysis solutions brought me the efficiency and enabled me to focus on result interpretation for my research program. I am confident in continuing work with the biocrates team for my discoveries in future studies.”
Related assets
brochure
Pharma
pdf ~ 4 MB
download
brochure
biocrates kit technology
pdf ~ 2 MB
download
brochure
biocrates services
pdf ~ 1.5 MB
download
brochure
WebIDQ software
pdf ~ 2 MB
download
Talk to an expert

biocrates life sciences gmbh
Eduard-Bodem-Gasse 8
6020 Innsbruck | AUSTRIA
Phone +43 512 579823
Fax +43 512 579823-329
biocrates inc.
26895 Aliso Creek Rd Ste B-426
Aliso Viejo, CA 92656-5301
Phone +1 774 424 8150
Operating hours: Mo – Fr | 9 am – 7 pm (EST)